The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases
- PMID: 38374298
- PMCID: PMC11216899
- DOI: 10.1038/s41577-024-00994-x
The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases
Abstract
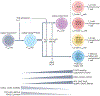
The discovery of FOXP3+ regulatory T (Treg) cells as a distinct cell lineage with a central role in regulating immune responses provided a deeper understanding of self-tolerance. The transcription factor FOXP3 serves a key role in Treg cell lineage determination and maintenance, but is not sufficient to enable the full potential of Treg cell suppression, indicating that other factors orchestrate the fine-tuning of Treg cell function. Moreover, FOXP3-independent mechanisms have recently been shown to contribute to Treg cell dysfunction. FOXP3 mutations in humans cause lethal fulminant systemic autoinflammation (IPEX syndrome). However, it remains unclear to what degree Treg cell dysfunction is contributing to the pathophysiology of common autoimmune diseases. In this Review, we discuss the origins of Treg cells in the periphery and the multilayered mechanisms by which Treg cells are induced, as well as the FOXP3-dependent and FOXP3-independent cellular programmes that maintain the suppressive function of Treg cells in humans and mice. Further, we examine evidence for Treg cell dysfunction in the context of common autoimmune diseases such as multiple sclerosis, inflammatory bowel disease, systemic lupus erythematosus and rheumatoid arthritis.
© 2024. Springer Nature Limited.
Figures

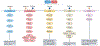
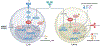
References
-
- Miyara M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

