Analytical characterization of full, intermediate, and empty AAV capsids
- PMID: 38374348
- PMCID: PMC11090809
- DOI: 10.1038/s41434-024-00444-2
Analytical characterization of full, intermediate, and empty AAV capsids
Abstract
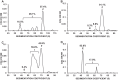
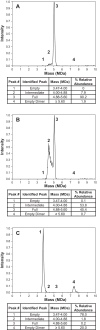
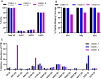
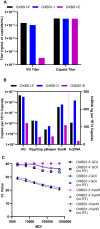
Manufacturing of recombinant adeno-associated virus (AAV) vectors produces three types of capsids: full, intermediate, and empty. While there are different opinions about the impact of intermediate and empty capsids on safety and efficacy of AAV products, they are generally considered impurities because they are not the intended fully intact vector product. The presence of these impurities could impact product efficacy due to potential competition with fully packaged AAVs for cellular transduction, as well as have potential implications to patient safety due to increased capsid load during dosing. To determine the impact of intermediate capsids on potency, an AAV preparation was separated into fractions enriched for full, intermediate, or empty capsids. Using a matrix of in vitro (infectivity, gene expression, biological activity) and in vivo potency assays to determine potency as a function of capsid content, our results indicate that while intermediate capsids contribute to the vector genome titer of the product and are equally as infectious as full capsids, they do not contribute to the potency of the AAV product. This study confirms the criticality of reducing and controlling the level of intermediate capsids to ensure a more efficacious AAV product.
© 2024. The Author(s).
Conflict of interest statement
All authors have received salary with employee stock options and/or restricted stock units during their employment at Oxford Biomedica (US) LLC, except for Robert Bruccoleri (Congenomics, LLC), who is a paid consultant for Oxford Biomedica (US) LLC. None of the authors have competing financial interests in relation to the work described.
Figures

References
-
- Van Lieshout LP, Rubin M, Costa-Grant K, Ota S, Golebiowski D, Panico T, et al. A novel dual-plasmid platform provides scalable transfection yielding improved productivity and packaging across multiple AAV serotypes and genomes. Mol Therapy Methods Clin Dev. 2023;29:426–36. doi: 10.1016/j.omtm.2023.05.004. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

