Pseudorabies virus uses clathrin mediated endocytosis to enter PK15 swine cell line
- PMID: 38374920
- PMCID: PMC10876092
- DOI: 10.3389/fmicb.2024.1332175
Pseudorabies virus uses clathrin mediated endocytosis to enter PK15 swine cell line
Abstract
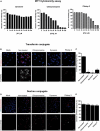
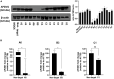
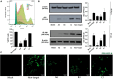
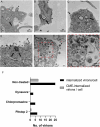
Pseudorabies virus (PRV), a herpesvirus responsible for Aujeszky's disease, causes high mortality in swine populations. To develop effective and novel antiviral strategies, it is essential to understand the mechanism of entry used by PRV to infect its host. Viruses have different ways of entering host cells. Among others, they can use endocytosis, a fundamental cellular process by which substances from the external environment are internalized into the cell. This process is classified into clathrin-mediated endocytosis (CME) and clathrin-independent endocytosis (CIE), depending on the role of clathrin. Although the involvement of cholesterol-rich lipid rafts in the entry of PRV has already been described, the importance of other endocytic pathways involving clathrin remains unexplored to date. Here, we characterize the role of CME in PRV entry into the PK15 swine cell line. By using CME inhibitory drugs, we report a decrease in PRV infection when the CME pathway is blocked. We also perform the shRNA knockdown of the μ-subunit of the adaptor protein AP-2 (AP2M1), which plays an important role in the maturation of clathrin-coated vesicles, and the infection is greatly reduced when this subunit is knocked down. Furthermore, transmission electron microscopy images report PRV virions inside clathrin-coated vesicles. Overall, this study suggests for the first time that CME is a mechanism used by PRV to enter PK15 cells and provides valuable insights into its possible routes of entry.
Keywords: PK15 cell line; clathrin; herpesvirus; pseudorabies virus; viral entry.
Copyright © 2024 Andreu, Agúndez,, López-Guerrero and Bello-Morales.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Human Coronavirus 229E Uses Clathrin-Mediated Endocytosis as a Route of Entry in Huh-7 Cells.Biomolecules. 2024 Sep 29;14(10):1232. doi: 10.3390/biom14101232. Biomolecules. 2024. PMID: 39456165 Free PMC article.
-
Low-pH Endocytic Entry of the Porcine Alphaherpesvirus Pseudorabies Virus.J Virol. 2019 Jan 4;93(2):e01849-18. doi: 10.1128/JVI.01849-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355685 Free PMC article.
-
Pseudorabies Virus Inhibits Expression of Liver X Receptors to Assist Viral Infection.Viruses. 2022 Mar 3;14(3):514. doi: 10.3390/v14030514. Viruses. 2022. PMID: 35336921 Free PMC article.
-
Revisiting Pathogen Exploitation of Clathrin-Independent Endocytosis: Mechanisms and Implications.Cells. 2025 May 16;14(10):731. doi: 10.3390/cells14100731. Cells. 2025. PMID: 40422234 Free PMC article. Review.
-
Involvement of adaptor proteins in clathrin-mediated endocytosis of virus entry.Microb Pathog. 2021 Dec;161(Pt A):105278. doi: 10.1016/j.micpath.2021.105278. Epub 2021 Nov 2. Microb Pathog. 2021. PMID: 34740810 Review.
Cited by
-
Immune Modulatory Effects of Vitamin D on Herpesvirus Infections.Int J Mol Sci. 2025 Feb 19;26(4):1767. doi: 10.3390/ijms26041767. Int J Mol Sci. 2025. PMID: 40004230 Free PMC article. Review.
-
Human Coronavirus 229E Uses Clathrin-Mediated Endocytosis as a Route of Entry in Huh-7 Cells.Biomolecules. 2024 Sep 29;14(10):1232. doi: 10.3390/biom14101232. Biomolecules. 2024. PMID: 39456165 Free PMC article.
-
Alphaherpesvirus in Pets and Livestock.Microorganisms. 2025 Jan 4;13(1):82. doi: 10.3390/microorganisms13010082. Microorganisms. 2025. PMID: 39858850 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

