All-in-one IQ toggle switches with high versatilities for fine-tuning of transgene expression in mammalian cells and tissues
- PMID: 38374964
- PMCID: PMC10875299
- DOI: 10.1016/j.omtm.2024.101202
All-in-one IQ toggle switches with high versatilities for fine-tuning of transgene expression in mammalian cells and tissues
Abstract
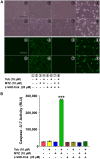
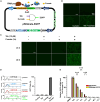
The transgene toggling device is recognized as a powerful tool for gene- and cell-based biological research and precision medicine. However, many of these devices often operate in binary mode, exhibit unacceptable leakiness, suffer from transgene silencing, show cytotoxicity, and have low potency. Here, we present a novel transgene switch, SIQ, wherein all the elements for gene toggling are packed into a single vector. SIQ has superior potency in inducing transgene expression in response to tebufenozide compared with the Gal4/UAS system, while completely avoiding transgene leakiness. Additionally, the ease and versatility of SIQ make it possible with a single construct to perform transient transfection, establish stable cell lines by targeting a predetermined genomic locus, and simultaneously produce adenovirus for transduction into cells and mammalian tissues. Furthermore, we integrated a cumate switch into SIQ, called SIQmate, to operate a Boolean AND logic gate, enabling swift toggling-off of the transgene after the removal of chemical inducers, tebufenozide and cumate. Both SIQ and SIQmate offer precise transgene toggling, making them adjustable for various researches, including synthetic biology, genome engineering, and therapeutics.
Keywords: SIQ; SIQmate; adenovirus; cumate; phiC31; tebufenozide; transgene toggling.
© 2024.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
An Ecdysone Receptor-based Singular Gene Switch for Deliberate Expression of Transgene with Robustness, Reversibility, and Negligible Leakiness.J Vis Exp. 2018 May 7;(135):57494. doi: 10.3791/57494. J Vis Exp. 2018. PMID: 29781995 Free PMC article.
-
Ecdysone Receptor-based Singular Gene Switches for Regulated Transgene Expression in Cells and Adult Rodent Tissues.Mol Ther Nucleic Acids. 2016 Sep 27;5(9):e367. doi: 10.1038/mtna.2016.74. Mol Ther Nucleic Acids. 2016. PMID: 27673563 Free PMC article.
-
IQ-Switch is a QF-based innocuous, silencing-free, and inducible gene switch system in zebrafish.Commun Biol. 2021 Dec 16;4(1):1405. doi: 10.1038/s42003-021-02923-3. Commun Biol. 2021. PMID: 34916605 Free PMC article.
-
Gal4 Driver Transgenic Zebrafish: Powerful Tools to Study Developmental Biology, Organogenesis, and Neuroscience.Adv Genet. 2016;95:65-87. doi: 10.1016/bs.adgen.2016.04.002. Epub 2016 Jun 13. Adv Genet. 2016. PMID: 27503354 Review.
-
Mammalian synthetic biology: engineering of sophisticated gene networks.J Biotechnol. 2007 Jul 15;130(4):329-45. doi: 10.1016/j.jbiotec.2007.05.014. Epub 2007 May 24. J Biotechnol. 2007. PMID: 17602777 Review.
Cited by
-
There and turn back again: the application of phage serine integrases in eukaryotic systems.Front Bioeng Biotechnol. 2025 Feb 24;13:1478413. doi: 10.3389/fbioe.2025.1478413. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40066361 Free PMC article. Review.
References
-
- Urlinger S., Baron U., Thellmann M., Hasan M.T., Bujard H., Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

