Comparison of remimazolam and propofol combined with low dose esketamine for pediatric same-day painless bidirectional endoscopy: a randomized, controlled clinical trial
- PMID: 38375038
- PMCID: PMC10875078
- DOI: 10.3389/fphar.2024.1298409
Comparison of remimazolam and propofol combined with low dose esketamine for pediatric same-day painless bidirectional endoscopy: a randomized, controlled clinical trial
Abstract
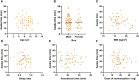
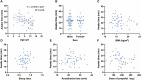
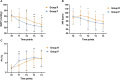
Background: Remimazolam has shown similar or even superior properties to propofol in procedural sedation in adults, but few studies have been conducted in pediatric populations. Thus, we aimed to compare the effect and safety of remimazolam and propofol combined with low dose esketamine for pediatric same-day bidirectional endoscopy (BDE). Methods: Pediatrics <18 years scheduled for elective BDE under sedation were included and randomly assigned to remimazolam group (R group) or propofol group (P group). The primary outcome was the success rate of sedation. Secondary outcomes include sedation-related information and adverse events. Mean arterial pressure (MAP), heart rate (HR), and perfusion index (PI) were recorded during sedation. Results: A total of 106 patients were enrolled and analyzed. The success rate of sedation was 100% in both groups. Compared with the P group, the induction time of the R group was significantly prolonged (p < 0.001), and the incidence of injection pain, intraoperative respiratory depression, hypotension and bradycardia was significantly lower (p < 0.001). The changes in MAP, HR and PI were relatively stable in the R group compared with the P group. Additionally, awake time significantly decreased with age by approximately 1.12 index points for each increase in age in the P group (p = 0.002) but not in the R group (p > 0.05). Furthermore, the decline in PI and PI ratio during BDE was related to body movement in the P group. Conclusion: Remimazolam combined with low dose esketamine has a non-inferior sedative effect than propofol for pediatric BDE, with no injection pain, less respiratory depression, more stable hemodynamics. Moreover, early detection of the decline in PI may avoid harmful stimulation under light anesthesia. Clinical trial registration: https://www.clinicaltrials.gov/study/NCT05686863?id=NCT05686863&rank=1, NCT05686863.
Keywords: bidirectional endoscopy; esketamine; pediatric; propofol; remimazolam; sedation.
Copyright © 2024 Chu, Zhou, Wan, Liu, Xin, Tian, Yan and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Comparative Analysis of Hemodynamic Effects of Remimazolam and Propofol Combined with Esketamine in Colonoscopic Procedures in the Elderly.Drug Des Devel Ther. 2024 Nov 19;18:5269-5280. doi: 10.2147/DDDT.S490179. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39588393 Free PMC article. Clinical Trial.
-
Remimazolam Tosylate Combined with Low-Dose Propofol Improves Sedation and Safety in Hysteroscopy.Drug Des Devel Ther. 2022 Nov 29;16:4101-4108. doi: 10.2147/DDDT.S390403. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36471692 Free PMC article. Clinical Trial.
-
The efficacy and safety of remimazolam in painless colonoscopy: a prospective, randomized clinical trial.Front Med (Lausanne). 2024 Nov 20;11:1434767. doi: 10.3389/fmed.2024.1434767. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39635591 Free PMC article.
-
Comparison of the safety of remimazolam and propofol during general anesthesia in elderly patients: systematic review and meta-analysis.Front Med (Lausanne). 2025 Feb 7;12:1409495. doi: 10.3389/fmed.2025.1409495. eCollection 2025. Front Med (Lausanne). 2025. PMID: 39991057 Free PMC article.
-
Remimazolam versus propofol for procedural sedation and anesthesia: a systemic review and meta-analysis.Minerva Anestesiol. 2022 Dec;88(12):1035-1042. doi: 10.23736/S0375-9393.22.16817-3. Epub 2022 Nov 3. Minerva Anestesiol. 2022. PMID: 36326772
Cited by
-
The Influence of Age on the Effective Dosage of Intravenous Remimazolam for the Relief of Preoperative Anxiety in Pediatric Patients at Median and 95% Effective Doses: A Prospective Study.Drug Des Devel Ther. 2025 Jun 1;19:4605-4615. doi: 10.2147/DDDT.S515924. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40486122 Free PMC article. Clinical Trial.
-
Remimazolam in General Anesthesia: A Comprehensive Review of Its Applications and Clinical Efficacy.Drug Des Devel Ther. 2024 Aug 5;18:3487-3498. doi: 10.2147/DDDT.S474854. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39132624 Free PMC article. Review.
-
The 50% effective dose of remimazolam combined with different doses of esketamine for painless gastroscopy.Sci Rep. 2025 Apr 14;15(1):12770. doi: 10.1038/s41598-025-97649-1. Sci Rep. 2025. PMID: 40229355 Free PMC article. Clinical Trial.
References
-
- Borkett K. M., Riff D. S., Schwartz H. I., Winkle P. J., Pambianco D. J., Lees J. P., et al. (2015). A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth. Analg. 120 (4), 771–780. 10.1213/ANE.0000000000000548 - DOI - PubMed
-
- Chen S. L., Zhu X., Zhao J. F., Zhai X. Q., Li J., Chang P. A. (2022). The effect of esketamine on peri-examination stress response in patients undergoing painless gastrointestinal endoscopy. China&Foreign Med. Treat. 41 (14), 14–18. 10.16662/j.cnki.1674-0742.2022.14.014 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

