A Bispecific, Tetravalent Antibody Targeting Inflammatory and Pruritogenic Pathways in Atopic Dermatitis
- PMID: 38375189
- PMCID: PMC10875227
- DOI: 10.1016/j.xjidi.2024.100258
A Bispecific, Tetravalent Antibody Targeting Inflammatory and Pruritogenic Pathways in Atopic Dermatitis
Abstract
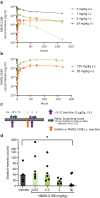
Inhibition of IL-4/IL-13 signaling has dramatically improved the treatment of atopic dermatitis (AD). However, in many patients, clinical responses are slow to develop and remain modest. Indeed, some symptoms of AD are dependent on IL-31, which is only partially reduced by IL-4/IL-13 inhibition. Thus, there is an unmet need for AD treatments that concomitantly block IL-4/IL-13 and IL-31 pathways. We engineered NM26-2198, a bispecific tetravalent antibody designed to accomplish this task. In reporter cell lines, NM26-2198 concomitantly inhibited IL-4/IL-13 and IL-31 signaling with a potency comparable with that of the combination of an anti-IL-4Rα antibody (dupilumab) and an anti-IL-31 antibody (BMS-981164). In human PBMCs, NM26-2198 inhibited IL-4-induced upregulation of CD23, demonstrating functional binding to FcγRII (CD32). NM26-2198 also inhibited the secretion of the AD biomarker thymus and activation-regulated chemokine (TARC) in blood samples from healthy human donors. In male cynomolgus monkeys, NM26-2198 exhibited favorable pharmacokinetics and significantly inhibited IL-31-induced scratching at a dose of 30 mg/kg. In a repeat-dose, good laboratory practice toxicology study in cynomolgus monkeys, no adverse effects of NM26-2198 were observed at a weekly dose of 125 mg/kg. Together, these results justify the clinical investigation of NM26-2198 as a treatment for moderate-to-severe AD.
Keywords: Atopic dermatitis; Cytokines; Inflammatory skin diseases; Pruritus.
© 2024 The Authors.
Figures



References
-
- Bieber T., Simpson E.L., Silverberg J.I., Thaçi D., Paul C., Pink A.E., et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101–1112. - PubMed
-
- Bitton A., Avlas S., Reichman H., Itan M., Karo-Atar D., Azouz N.P., et al. A key role for IL-13 signaling via the type 2 IL-4 receptor in experimental atopic dermatitis. Sci Immunol. 2020;5 - PubMed
-
- Blauvelt A., de Bruin-Weller M., Gooderham M., Cather J.C., Weisman J., Pariser D., et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (Liberty AD Chronos): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

