Predictable regulation of survival by intratumoral microbe-immune crosstalk in patients with lung adenocarcinoma
- PMID: 38375207
- PMCID: PMC10876218
- DOI: 10.15698/mic2024.02.813
Predictable regulation of survival by intratumoral microbe-immune crosstalk in patients with lung adenocarcinoma
Abstract
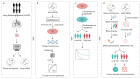
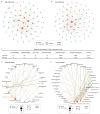
Intratumoral microbiota can regulate the tumor immune microenvironment (TIME) and mediate tumor prognosis by promoting inflammatory response or inhibiting anti-tumor effects. Recent studies have elucidated the potential role of local tumor microbiota in the development and progression of lung adenocarcinoma (LUAD). However, whether intratumoral microbes are involved in the TIME that mediates the prognosis of LUAD remains unknown. Here, we obtained the matched tumor microbiome and host transcriptome and survival data of 478 patients with LUAD in The Cancer Genome Atlas (TCGA). Machine learning models based on immune cell marker genes can predict 1- to 5-year survival with relative accuracy. Patients were stratified into high- and low-survival-risk groups based on immune cell marker genes, with significant differences in intratumoral microbial communities. Specifically, patients in the high-risk group had significantly higher alpha diversity (p < 0.05) and were characterized by an enrichment of lung cancer-related genera such as Streptococcus. However, network analysis highlighted a more active pattern of dominant bacteria and immune cell crosstalk in TIME in the low-risk group compared to the high-risk group. Our study demonstrated that intratumoral microbiota-immune crosstalk was strongly associated with prognosis in LUAD patients, which would provide new targets for the development of precise therapeutic strategies.
Keywords: immune cell; intratumoral microbiota; lung adenocarcinoma; prognosis; tumor microenvironment.
Copyright: © 2024 Shi et al.
Conflict of interest statement
Conflict of Interest: The authors declare that they have no known competing financial interests or personal relationships.
Figures



Similar articles
-
Intratumor microbiome derived glycolysis-lactate signatures depicts immune heterogeneity in lung adenocarcinoma by integration of microbiomic, transcriptomic, proteomic and single-cell data.Front Microbiol. 2023 Aug 17;14:1202454. doi: 10.3389/fmicb.2023.1202454. eCollection 2023. Front Microbiol. 2023. PMID: 37664112 Free PMC article.
-
The Interaction between Intratumoral Microbiome and Immunity Is Related to the Prognosis of Ovarian Cancer.Microbiol Spectr. 2023 Mar 28;11(2):e0354922. doi: 10.1128/spectrum.03549-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36975828 Free PMC article.
-
Bidirectional Mediation Effects between Intratumoral Microbiome and Host DNA Methylation Changes Contribute to Stomach Adenocarcinoma.Microbiol Spectr. 2023 Aug 17;11(4):e0090423. doi: 10.1128/spectrum.00904-23. Epub 2023 Jun 1. Microbiol Spectr. 2023. PMID: 37260411 Free PMC article.
-
Crosstalk Between the Intratumoral Microbiota and the Tumor Microenvironment: New Frontiers in Solid Tumor Progression and Treatment.Cancer Rep (Hoboken). 2024 Nov;7(11):e70063. doi: 10.1002/cnr2.70063. Cancer Rep (Hoboken). 2024. PMID: 39559964 Free PMC article. Review.
-
Spatial Heterogeneity of Intratumoral Microbiota: A New Frontier in Cancer Immunotherapy Resistance.Biomedicines. 2025 May 21;13(5):1261. doi: 10.3390/biomedicines13051261. Biomedicines. 2025. PMID: 40427087 Free PMC article. Review.
Cited by
-
Worldwide Research Trends on Lung Cancer and Microbiota: A Bibliometric and Visualized Analysis.J Multidiscip Healthc. 2025 Jul 29;18:4291-4308. doi: 10.2147/JMDH.S516036. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 40761583 Free PMC article. Review.
-
Unlocking the Microbial Symphony: The Interplay of Human Microbiota in Cancer Immunotherapy Response.Cancers (Basel). 2025 Feb 26;17(5):813. doi: 10.3390/cancers17050813. Cancers (Basel). 2025. PMID: 40075661 Free PMC article. Review.
-
Intra-tumor microbiome-based tumor survival indices predict immune interaction and drug sensitivity on pan-cancer scale.mSystems. 2025 Jul 22;10(7):e0031225. doi: 10.1128/msystems.00312-25. Epub 2025 Jun 25. mSystems. 2025. PMID: 40558028 Free PMC article.
-
Artificial intelligence in lung cancer: current applications, future perspectives, and challenges.Front Oncol. 2024 Dec 23;14:1486310. doi: 10.3389/fonc.2024.1486310. eCollection 2024. Front Oncol. 2024. PMID: 39763611 Free PMC article. Review.
-
Exploring micronutrients and microbiome synergy: pioneering new paths in cancer therapy.Front Immunol. 2024 Nov 29;15:1442788. doi: 10.3389/fimmu.2024.1442788. eCollection 2024. Front Immunol. 2024. PMID: 39676876 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
