Scaling up production of recombinant human basic fibroblast growth factor in an Escherichia coli BL21(DE3) plysS strain and evaluation of its pro-wound healing efficacy
- PMID: 38375209
- PMCID: PMC10875678
- DOI: 10.3389/fphar.2023.1279516
Scaling up production of recombinant human basic fibroblast growth factor in an Escherichia coli BL21(DE3) plysS strain and evaluation of its pro-wound healing efficacy
Abstract
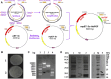
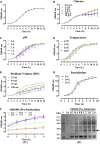
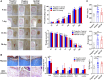
Introduction: Human basic fibroblast growth factor (hbFGF) is a highly valuable multifunctional protein that plays a crucial role in various biological processes. In this study, we aim to accomplish the scaling-up production of mature hbFGF (146aa) by implementing a high cell-density fermentation and purification process on a 500-L scale, thereby satisfying the escalating demands for both experimental research and clinical applications. Methods: The hbFGF DNA fragment was cloned into a mpET-3c vector containing a kanamycin resistance gene and then inserted into Escherichia coli BL21 (DE3) plysS strain. To optimize the yield of hbFGF protein, various fermentation parameters were systematically optimized using BOX-Behnken design and further validated in large-scale fermentation (500-L). Additionally, a three-step purification protocol involving CM-Sepharose, heparin affinity, and SP-Sepharose column chromatography was developed to separate and purify the hbFGF protein. Isoelectric focusing electrophoresis, MALDI-TOF/MS analysis, amino acid sequencing, CD spectroscopy, and Western blotting were performed to authenticate its identity. The biological efficacy of purified hbFGF was evaluated using an MTT assay as well as in a diabetic deep second-degree scald model. Results: The engineered strain was successfully constructed, exhibiting high expression of hbFGF and excellent stability. Under the optimized fermentation conditions, an impressive bacterial yield of 46.8 ± 0.3 g/L culture with an expression level of hbFGF reaching 28.2% ± 0.2% was achieved in 500-L scale fermentation. Subsequently, during pilot-scale purification, the final yield of purified hbFGF protein was 114.6 ± 5.9 mg/L culture with RP-HPLC, SEC-HPLC, and SDS-PAGE purity exceeding 98%. The properties of purified hbFGF including its molecular weight, isoelectric point (pI), amino sequence, and secondary structure were found to be consistent with theoretical values. Furthermore, the purified hbFGF exhibited potent mitogenic activity with a specific value of 1.05 ± 0.94 × 106 AU/mg and significantly enhanced wound healing in a deep second-degree scald wound diabetic rat model. Conclusion: This study successfully established a stable and efficient large-scale production process of hbFGF, providing a solid foundation for future industrial production.
Keywords: 500-L fermentation; Escherichia coli BL21(DE3) plysS; hbFGF; optimized production; purification; wound healing.
Copyright © 2024 Li, Yu, Lai, Shen, Yan, Dong, Gao, Cao, Ge, Zhu, Liu, Tao, Yao, Li, Wang and Hui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Akl M. R., Nagpal P., Ayoub N. M., Tai B., Prabhu S. A., Capac C. M., et al. (2016). Molecular and clinical significance of fibroblast growth factor 2 (FGF2/bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 7 (28), 44735–44762. 10.18632/oncotarget.8203 - DOI - PMC - PubMed
-
- Bai F. Y., Zhao B., Li Y. J., Gao Y., Liu Q. (2002). Study on fermentation process of recombinant human basic fibroblast growth factor expressed in Escherichia coli . Lett. Biotechnol. 04, 278–280.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

