The mechanisms and therapeutic potential of clopidogrel in mitigating diabetic cardiomyopathy in db/db mice
- PMID: 38375215
- PMCID: PMC10875154
- DOI: 10.1016/j.isci.2024.109134
The mechanisms and therapeutic potential of clopidogrel in mitigating diabetic cardiomyopathy in db/db mice
Abstract
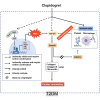
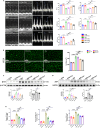
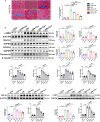
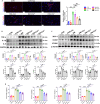
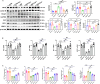
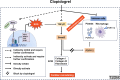
Clopidogrel has been shown to play a protective role against diabetic nephropathy. However, whether clopidogrel exerts a protective effect against diabetic cardiomyopathy (DCM) is unknown. Three-month-old male db/db mice were administered clopidogrel daily at doses of 5, 10, and 20 mg/kg by gavage for 5 months. Here, we showed that clopidogrel effectively attenuated diabetes-induced cardiac hypertrophy and cardiac dysfunction by inhibiting cardiac fibrosis, inflammatory responses, and oxidative stress damage in db/db mice. Diabetes-induced cardiac fibrosis was inhibited by clopidogrel treatment via blockade of the TGF-β1/Smad3/P2RY12 pathway and inhibition of macrophage infiltration in db/db mice. The protective effects of clopidogrel against oxidative damage were mediated by the induction of the Nrf2 signaling pathway. Taken together, our findings provide strong evidence that clopidogrel is a promising effective agent for the treatment of DCM by alleviating diabetes-induced cardiac hypertrophy and dysfunction. P2RY12 might be an effective target for the treatment of DCM.
Keywords: Biological sciences; Diabetology; Endocrinology; Natural sciences; Pathophysiology; Pharmacology; Physiology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Isoliquiritigenin attenuates diabetic cardiomyopathy via inhibition of hyperglycemia-induced inflammatory response and oxidative stress.Phytomedicine. 2020 Nov;78:153319. doi: 10.1016/j.phymed.2020.153319. Epub 2020 Sep 1. Phytomedicine. 2020. PMID: 32950951
-
Empagliflozin Ameliorates Diabetic Cardiomyopathy via Attenuating Oxidative Stress and Improving Mitochondrial Function.Oxid Med Cell Longev. 2022 May 9;2022:1122494. doi: 10.1155/2022/1122494. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35585884 Free PMC article.
-
Clopidogrel delays and can reverse diabetic nephropathy pathogenesis in type 2 diabetic db/db mice.World J Diabetes. 2022 Aug 15;13(8):600-612. doi: 10.4239/wjd.v13.i8.600. World J Diabetes. 2022. PMID: 36159226 Free PMC article.
-
FNDC5/Irisin attenuates diabetic cardiomyopathy in a type 2 diabetes mouse model by activation of integrin αV/β5-AKT signaling and reduction of oxidative/nitrosative stress.J Mol Cell Cardiol. 2021 Nov;160:27-41. doi: 10.1016/j.yjmcc.2021.06.013. Epub 2021 Jul 3. J Mol Cell Cardiol. 2021. PMID: 34224725
-
Obeticholic acid protects against diabetic cardiomyopathy by activation of FXR/Nrf2 signaling in db/db mice.Eur J Pharmacol. 2019 Sep 5;858:172393. doi: 10.1016/j.ejphar.2019.05.022. Epub 2019 May 11. Eur J Pharmacol. 2019. PMID: 31085240
Cited by
-
Sleep restriction exacerbates cardiac dysfunction in diabetic mice by causing cardiomyocyte death and fibrosis through mitochondrial damage.Cell Death Discov. 2024 Oct 21;10(1):446. doi: 10.1038/s41420-024-02214-w. Cell Death Discov. 2024. PMID: 39433752 Free PMC article.
References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9 (th) edition. Diabetes Res. Clin. Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

