The hippocampus contributes to retroactive stimulus associations during trace fear conditioning
- PMID: 38375237
- PMCID: PMC10875141
- DOI: 10.1016/j.isci.2024.109035
The hippocampus contributes to retroactive stimulus associations during trace fear conditioning
Abstract
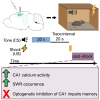
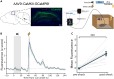
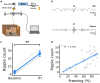
Binding events that occur at different times are essential for memory formation. In trace fear conditioning, animals associate a tone and footshock despite no temporal overlap. The hippocampus is thought to mediate this learning by maintaining a memory of the tone until shock occurrence, however, evidence for sustained hippocampal tone representations is lacking. Here, we demonstrate a retrospective role for the hippocampus in trace fear conditioning. Bulk calcium imaging revealed sustained increases in CA1 activity after footshock that were not observed after tone termination. Optogenetic silencing of CA1 immediately after footshock impaired subsequent memory. Additionally, footshock increased the number of sharp-wave ripples compared to baseline during conditioning. Therefore, post-shock hippocampal activity likely supports learning by reactivating and linking latent tone and shock representations. These findings highlight an underappreciated function of post-trial hippocampal activity in enabling retroactive temporal associations during new learning, as opposed to persistent maintenance of stimulus representations.
Keywords: Behavioral neuroscience; Cellular neuroscience; Neuroscience.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

