Biogenic Salvia species synthesized silver nanoparticles with catalytic, sensing, antimicrobial, and antioxidant properties
- PMID: 38375246
- PMCID: PMC10875438
- DOI: 10.1016/j.heliyon.2024.e25814
Biogenic Salvia species synthesized silver nanoparticles with catalytic, sensing, antimicrobial, and antioxidant properties
Abstract
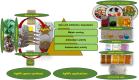
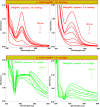
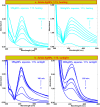
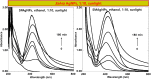
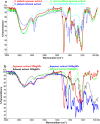
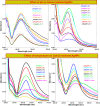
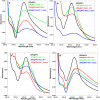
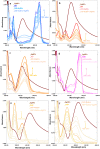
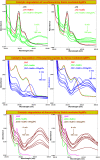
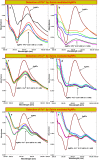
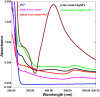
Salvia (Lamiaceae family) is used as a brain tonic to improve cognitive function. The species including S. plebeia and S. moorcroftiana are locally used to cure hepatitis, cough, tumours, hemorrhoids, diarrhoea, common cold, flu, and asthma. To the best of authors' knowledge, no previous study has been conducted on synthesis of S. plebeia and S. moorcroftiana silver nanoparticles (SPAgNPs and SMAgNPs). The study was aimed to synthesize AgNPs from the subject species aqueous and ethanol extracts, and assess catalytic potential in degradation of standard and extracted (from yums, candies, and snacks) dyes, nitrophenols, and antibiotics. The study also aimed at AgNPs as probe in sensing metalloids and heavy metal ions including Pb2+, Cu2+, Fe3+, Ni2+, and Zn2+. From the results, it was found that Salvia aqueous extract afforded stable AgNPs in 1:9 and 1:15 (quantity of aqueous extract and silver nitrate solution concentration) whereas ethanol extract yielded AgNPs in 1:10 (quantity of ethanol extract and silver nitrate solution concentration) reacted in sunlight. The size of SPAgNPs and SMAgNPs determined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were 21.7 nm and 19.9 nm, with spherical, cylindrical, and deep hollow morphology. The synthesized AgNPs demonstrated significant potential as catalyst in dyes; Congo red (85 %), methylene blue (75 %), Rhodamine B (<50 %), nitrophenols; ortho-nitrophenol (95-98 %) and para-nitrophenol (95-98 %), dyes extracted from food samples including yums, candies, and snacks. The antibiotics (amoxicillin, doxycycline, levofloxacin) degraded up to 80 %-95 % degradation. Furthermore, the synthesized AgNPs as probe in sensing of Pb2+, Cu2+, and Fe3+ in Kabul river water, due to agglomeration, caused a significant decrease and bathochromic shift of SPR band (430 nm) when analyzed after 30 min. The Pb2+ ions was comparatively more agglomerated and chelated. Thus, the practical applicability of AgNPs in Pb2+ sensing was significant. Based on the results of this research study, the synthesized AgNPs could provide promising efficiency in wastewater treatment containing organic dyes, antibiotics, and heavy metals.
Keywords: Antibiotics; Heavy metals; S. moorcroftiana; S. plebeia; Silver nanoparticles; Snacks.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Nargis Jamila reports financial support was provided by Higher Education Commission Pakistan.
Figures






References
LinkOut - more resources
Full Text Sources

