Effective γδ T-cell clinical therapies: current limitations and future perspectives for cancer immunotherapy
- PMID: 38375329
- PMCID: PMC10875631
- DOI: 10.1002/cti2.1492
Effective γδ T-cell clinical therapies: current limitations and future perspectives for cancer immunotherapy
Abstract
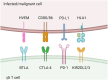
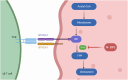
γδ T cells are a unique subset of T lymphocytes, exhibiting features of both innate and adaptive immune cells and are involved with cancer immunosurveillance. They present an attractive alternative to conventional T cell-based immunotherapy due, in large part, to their lack of major histocompatibility (MHC) restriction and ability to secrete high levels of cytokines with well-known anti-tumour functions. To date, clinical trials using γδ T cell-based immunotherapy for a range of haematological and solid cancers have yielded limited success compared with in vitro studies. This inability to translate the efficacy of γδ T-cell therapies from preclinical to clinical trials is attributed to a combination of several factors, e.g. γδ T-cell agonists that are commonly used to stimulate populations of these cells have limited cellular uptake yet rely on intracellular mechanisms; administered γδ T cells display low levels of tumour-infiltration; and there is a gap in the understanding of γδ T-cell inhibitory receptors. This review explores the discrepancy between γδ T-cell clinical and preclinical performance and offers viable avenues to overcome these obstacles. Using more direct γδ T-cell agonists, encapsulating these agonists into lipid nanocarriers to improve their pharmacokinetic and pharmacodynamic profiles and the use of combination therapies to overcome checkpoint inhibition and T-cell exhaustion are ways to bridge the gap between preclinical and clinical success. Given the ability to overcome these limitations, the development of a more targeted γδ T-cell agonist-checkpoint blockade combination therapy has the potential for success in clinical trials which has to date remained elusive.
Keywords: bisphosphonates; combination therapy; immunotherapy; lipid nanocarriers; nanomedicine; γδ T cells.
© 2024 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
γδ T Cells: The Ideal Tool for Cancer Immunotherapy.Cells. 2020 May 24;9(5):1305. doi: 10.3390/cells9051305. Cells. 2020. PMID: 32456316 Free PMC article. Review.
-
The Diverse Roles of γδ T Cells in Cancer: From Rapid Immunity to Aggressive Lymphoma.Cancers (Basel). 2021 Dec 9;13(24):6212. doi: 10.3390/cancers13246212. Cancers (Basel). 2021. PMID: 34944832 Free PMC article. Review.
-
The Role of Human γδ T Cells in Anti-Tumor Immunity and Their Potential for Cancer Immunotherapy.Cells. 2020 May 13;9(5):1206. doi: 10.3390/cells9051206. Cells. 2020. PMID: 32413966 Free PMC article. Review.
-
γδ T cells in cancer immunotherapy.Oncotarget. 2017 Jan 31;8(5):8900-8909. doi: 10.18632/oncotarget.13051. Oncotarget. 2017. PMID: 27823972 Free PMC article. Review.
-
γδ T cells: The potential role in liver disease and implications for cancer immunotherapy.J Leukoc Biol. 2022 Dec;112(6):1663-1668. doi: 10.1002/JLB.5MR0822-733RRR. Epub 2022 Sep 13. J Leukoc Biol. 2022. PMID: 36098208 Review.
Cited by
-
Mitochondrial transplantation sensitizes chemotherapy to inhibit tumor development by enhancing anti-tumor immunity.Cancer Biol Med. 2025 Jun 19;22(6):648-71. doi: 10.20892/j.issn.2095-3941.2024.0596. Cancer Biol Med. 2025. PMID: 40538008 Free PMC article.
-
Uncovering the mysteries of human gamma delta T cells: from origins to novel therapeutics.Front Immunol. 2025 Apr 10;16:1543454. doi: 10.3389/fimmu.2025.1543454. eCollection 2025. Front Immunol. 2025. PMID: 40276509 Free PMC article. Review.
-
Two Are Better than One: The Bi-Specific Antibody Mosunetuzumab Reveals an Improved Immune Response of Vγ9Vδ2 T Cells Targeting CD20 in Malignant B Cells in Comparison to the Mono-Specific Antibody Obinutuzumab.Int J Mol Sci. 2025 Jan 31;26(3):1262. doi: 10.3390/ijms26031262. Int J Mol Sci. 2025. PMID: 39941030 Free PMC article.
-
Potential Benefits of Adding Alendronate, Celecoxib, Itraconazole, Ramelteon, and Simvastatin to Endometrial Cancer Treatment: The EC5 Regimen.Curr Issues Mol Biol. 2025 Feb 26;47(3):153. doi: 10.3390/cimb47030153. Curr Issues Mol Biol. 2025. PMID: 40136407 Free PMC article.
-
γδT cells, a key subset of T cell for cancer immunotherapy.Front Immunol. 2025 Mar 28;16:1562188. doi: 10.3389/fimmu.2025.1562188. eCollection 2025. Front Immunol. 2025. PMID: 40226616 Free PMC article. Review.
References
-
- Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther 2019; 34: 20180032. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
