DEXTENZA versus Topical Steroid or Antihistamine Therapy for Treatment of Allergic Conjunctivitis
- PMID: 38375441
- PMCID: PMC10875166
- DOI: 10.2147/OPTH.S440840
DEXTENZA versus Topical Steroid or Antihistamine Therapy for Treatment of Allergic Conjunctivitis
Abstract
Purpose: To compare clinical outcomes and patient preference for the dexamethasone intracanalicular insert (DEX) versus topical loteprednol (LOT) or olopatadine (OLO) for the treatment of allergic conjunctivitis in a real-world model of allergen exposure.
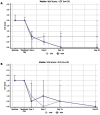
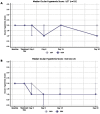
Methods: This was a prospective comparative trial. Adults with testing-confirmed bilateral allergic conjunctivitis received DEX in the more symptomatic eye and either LOT 2 times daily or OLO once daily for 30 days in the fellow eye. The primary outcome was patient preference for treatment. Clinical outcomes included ocular itching and hyperemia, lid swelling, and watering/tearing. Safety outcomes included intraocular pressure (IOP).
Results: Thirty patients participated and completed the study. All received DEX in the eye with worse symptoms and 15 received LOT and the other 15 received OLO in the other eye. Patients preferred DEX (10/15; 66.7%) over LOT (4/15; 26.7%), with one patient having no preference (p = 0.0103). Patients had no preference between DEX (8/15; 53.3%) and OLO (6/15; 40%), with one patient having no preference (p = 0.1044). In the DEX/LOT cohort, ocular itching and hyperemia improved more with DEX than LOT (p ≤ 0.009), while in the DEX/OLO cohort, the DEX eyes showed greater improvement in conjunctival hyperemia (p < 0.0001) but not itching (p = 0.074). No between-group differences were seen in eyelid swelling or tearing/watering in either cohort. Mean change in IOP was similar between the DEX and LOT eyes (p = 0.4921), and mean IOP rose more in the DEX eyes than the OLO eyes (by <1 mmHg; p = 0.0403).
Conclusion: Overall, this real-world study demonstrated that the dexamethasone intracanalicular insert was as effective as a topical antihistamine/mast cell stabilizer and more effective than topical steroids in relieving the signs and symptoms of allergic conjunctivitis. This insert should be considered as an alternative to topical therapy for the treatment of allergic conjunctivitis.
Keywords: allergic conjunctivitis; dexamethasone; dextenza.
© 2024 Reich et al.
Conflict of interest statement
Shani Reich MD reports funding to conduct this study and for editorial assistance provided by Ocular Therapeutix. The authors report no other conflicts of interest in this work.
Figures

References
-
- Virchow JC, Kay S, Demoly P, Mullol J, Canonica W, Higgins V. Impact of ocular symptoms on quality of life (QoL), work productivity and resource utilization in allergic rhinitis patients--an observational, cross sectional study in four countries in Europe. J Med Econ. 2011;14(3):305–314. doi: 10.3111/13696998.2011.576039 - DOI - PubMed
-
- Drugs@FDA: dextenza. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208742s007lbl.pdf. Accessed October 26, 2021.
Publication types
LinkOut - more resources
Full Text Sources

