Central nervous system immune-related disorders after SARS-CoV-2 vaccination: a multicenter study
- PMID: 38375477
- PMCID: PMC10876052
- DOI: 10.3389/fimmu.2024.1344184
Central nervous system immune-related disorders after SARS-CoV-2 vaccination: a multicenter study
Abstract
Background: COVID-19 vaccines have been approved due to their excellent safety and efficacy data and their use has also permitted to reduce neurological complications of SARS-CoV-2. However, clinical trials were underpowered to detect rare adverse events. Herein, the aim was to characterize the clinical spectrum and immunological features of central nervous system (CNS) immune-related events following SARS-CoV-2 vaccination.
Methods: Multicenter, retrospective, cohort study (December 1, 2020-April 30, 2022). Inclusion criteria were (1) de novo CNS disorders developing after SARS-CoV-2 vaccination (probable causal relationship as per 2021 Butler criteria) (2); evidence for an immune-mediated etiology, as per (i) 2016 Graus criteria for autoimmune encephalitis (AE); (ii) 2015 Wingerchuk criteria for neuromyelitis optica spectrum disorders; (iii) criteria for myelitis.
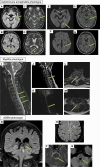
Results: Nineteen patients were included from 7 tertiary referral hospitals across Italy and France (one of them being a national referral center for AE), over almost 1 year and half of vaccination campaign. Vaccines administered were mRNA-based (63%) and adenovirus-vectored (37%). The median time between vaccination and symptoms onset was 14 days (range: 2-41 days). CSF was inflammatory in 74%; autoantibodies were detected in 5%. CSF cytokine analysis (n=3) revealed increased CXCL-10 (IP-10), suggesting robust T-cell activation. The patients had AE (58%), myelitis (21%), acute disseminated encephalomyelitis (ADEM) (16%), and brainstem encephalitis (5%). All patients but 2 received immunomodulatory treatment. At last follow-up (median 130 days; range: 32-540), only one patient (5%) had a mRS>2.
Conclusion: CNS adverse events of COVID-19 vaccination appear to be very rare even at reference centers and consist mostly of antibody-negative AE, myelitis, and ADEM developing approximately 2 weeks after vaccination. Most patients improve following immunomodulatory treatment.
Keywords: COVID-19; SARS-CoV-2; neurologic adverse events; neurological complications; vaccination; vaccine.
Copyright © 2024 Vogrig, Tartaglia, Dentoni, Fabris, Bax, Belluzzo, Verriello, Bagatto, Gastaldi, Tocco, Zoccarato, Zuliani, Pilotto, Padovani, Villagrán-García, Davy, Gigli, Honnorat and Valente.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Marchetti RL, Gallucci-Neto J, Kurcgant D, Proença ICGF, Valiengo L da CL, Fiore LA, et al. Immunization stress-related responses presenting as psychogenic non-epileptic seizures following HPV vaccination in Rio Branco, Brazil. Vaccine (2020) 38:6714–20. doi: 10.1016/j.vaccine.2020.08.044 - DOI - PubMed
-
- Butler M, Tamborska A, Wood GK, Ellul M, Thomas RH, Galea I, et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry (2021) 92:1144–51. doi: 10.1136/jnnp-2021-326924 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

