Dinuclear Zn-Catalytic System as Brønsted Base and Lewis Acid for Enantioselectivity in Same Chiral Environment
- PMID: 38375498
- PMCID: PMC10876046
- DOI: 10.1021/acsomega.3c07446
Dinuclear Zn-Catalytic System as Brønsted Base and Lewis Acid for Enantioselectivity in Same Chiral Environment
Abstract
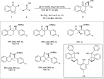
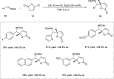
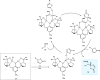
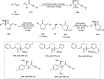
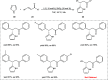
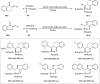
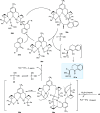
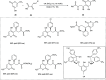
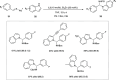
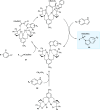
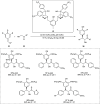
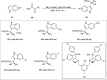
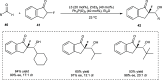
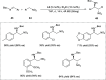
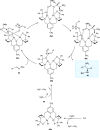
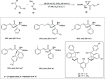
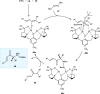
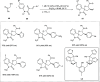
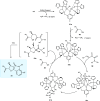
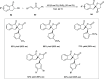
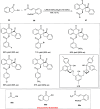
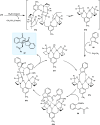
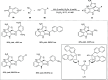
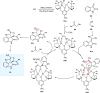
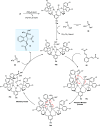
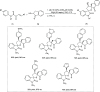
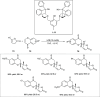
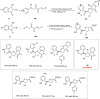
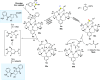
Zinc (Zn) is a crucial element with remarkable significance in organic transformations. The profusion of harmless zinc salts in the Earth's outer layer qualifies zinc as a noteworthy contender for inexpensive and eco-friendly reagents and catalysts. Recently, widely recognized uses of organo-Zn compounds in the field of organic synthesis have undergone extensive expansion toward asymmetric transformations. The ProPhenol ligand, a member of the chiral nitrogenous-crown family, exhibits the spontaneous formation of a dual-metal complex when reacted with alkyl metal (R-M) reagents, e.g., ZnEt2. The afforded Zn complex possesses two active sites, one Lewis acid and the other Brønsted base, thereby facilitating the activation of nucleophiles and electrophiles simultaneously within the same chiral pocket. In this comprehensive analysis, we provide a thorough account of the advancement and synthetic potential of these diverse catalysts in organic synthesis, while emphasizing the reactivity and selectivities, i.e., dr and ee due to the design/structure of the ligands employed.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
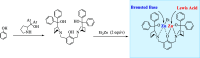
Similar articles
-
Dinuclear Metal-ProPhenol Catalysts: Development and Synthetic Applications.Angew Chem Int Ed Engl. 2020 Mar 9;59(11):4240-4261. doi: 10.1002/anie.201909692. Epub 2019 Nov 18. Angew Chem Int Ed Engl. 2020. PMID: 31452294 Review.
-
Cooperative Asymmetric Cation-Binding Catalysis.Acc Chem Res. 2021 Dec 7;54(23):4319-4333. doi: 10.1021/acs.accounts.1c00400. Epub 2021 Nov 16. Acc Chem Res. 2021. PMID: 34784182
-
Asymmetric functional organozinc additions to aldehydes catalyzed by 1,1'-bi-2-naphthols (BINOLs).Acc Chem Res. 2014 May 20;47(5):1523-35. doi: 10.1021/ar500020k. Epub 2014 Apr 16. Acc Chem Res. 2014. PMID: 24738985 Free PMC article. Review.
-
Brønsted-acid-catalyzed asymmetric multicomponent reactions for the facile synthesis of highly enantioenriched structurally diverse nitrogenous heterocycles.Acc Chem Res. 2011 Nov 15;44(11):1156-71. doi: 10.1021/ar2000343. Epub 2011 Jul 29. Acc Chem Res. 2011. PMID: 21800828
-
Low-oxidation state indium-catalyzed C-C bond formation.Acc Chem Res. 2012 Aug 21;45(8):1331-44. doi: 10.1021/ar300008t. Epub 2012 May 24. Acc Chem Res. 2012. PMID: 22626010 Review.
References
-
- Rudnick R. L.; Fountain D. M. Nature and composition of the continental crust: a lower crustal perspective. Reviews of geophysics 1995, 33 (3), 267–309. 10.1029/95RG01302. - DOI
- Rosman K. A survey of the isotopic and elemental abundance of zinc. Geochim. Cosmochim. Acta 1972, 36 (7), 801–819. 10.1016/0016-7037(72)90089-0. - DOI
- Li W.; Gou W.; Li W.; Zhang T.; Yu B.; Liu Q.; Shi J. Environmental applications of metal stable isotopes: Silver, mercury and zinc. Environ. Pollut. 2019, 252, 1344–1356. 10.1016/j.envpol.2019.06.037. - DOI - PubMed
-
- Hamer W. J.Standard cells: Their construction, maintenance, and characteristics; US Government Printing Office, 1965.
- Hall G. E.Purification and Biochemical Characterization of Carbonic Anhydrase from Erythrocytes of Salmo Gairdneri; The Pennsylvania State University, 1982.
-
- Busacca C. A.; Fandrick D. R.; Song J. J.; Senanayake C. H. The growing impact of catalysis in the pharmaceutical industry. Advanced Synthesis & Catalysis 2011, 353 (11–12), 1825–1864. 10.1002/adsc.201100488. - DOI
- Wender P. A.; Verma V. A.; Paxton T. J.; Pillow T. H. Function-Oriented Synthesis, Step Economy, and Drug Design. Acc. Chem. Res. 2008, 41, 40–49. 10.1021/ar700155p. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
