A novel interaction between aquaporin 1 and caspase-3 in pulmonary arterial smooth muscle cells
- PMID: 38375595
- PMCID: PMC11380937
- DOI: 10.1152/ajplung.00017.2024
A novel interaction between aquaporin 1 and caspase-3 in pulmonary arterial smooth muscle cells
Abstract
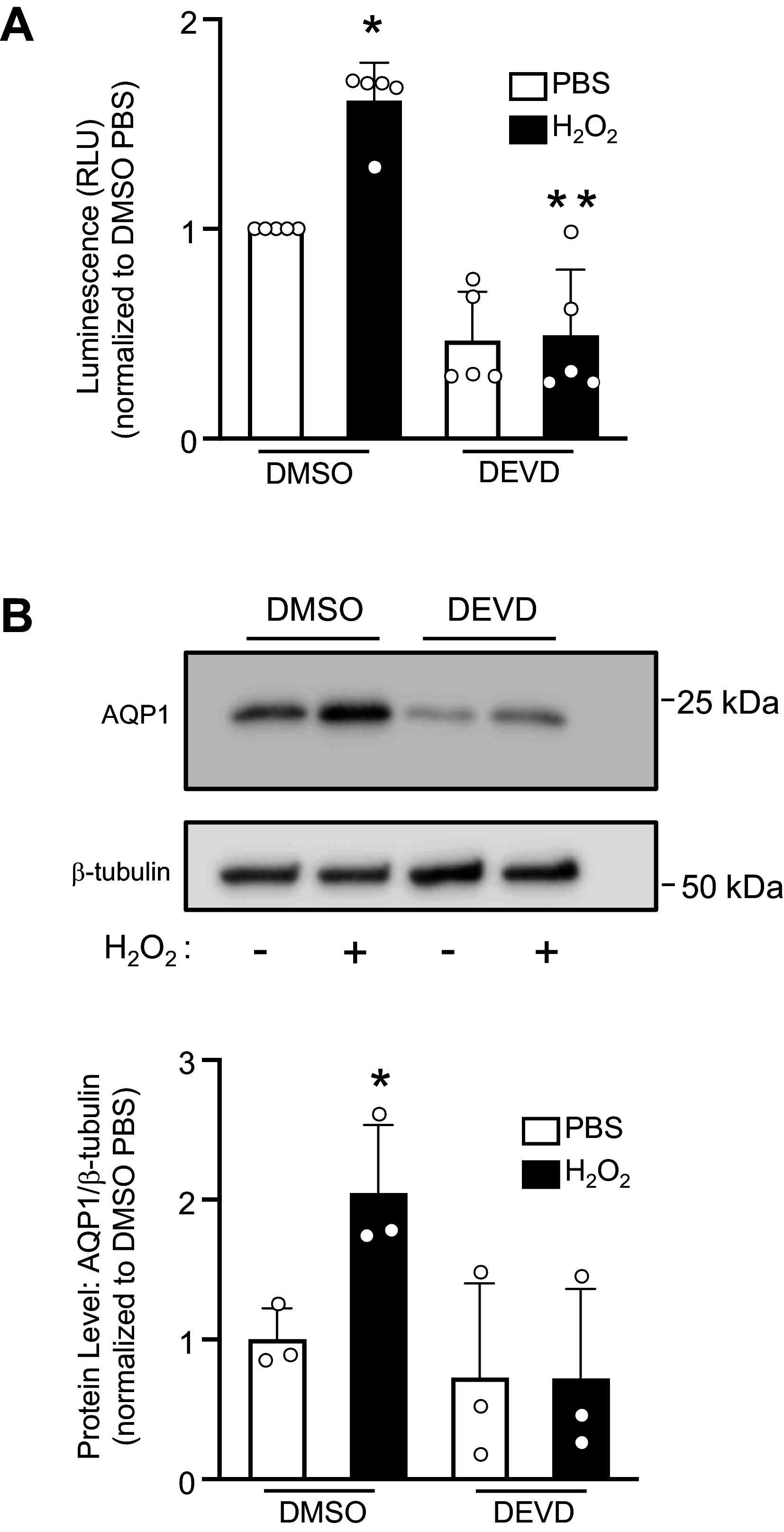
Pulmonary hypertension (PH) is a condition in which remodeling of the pulmonary vasculature leads to hypertrophy of the muscular vascular wall and extension of muscle into nonmuscular arteries. These pathological changes are predominantly due to the abnormal proliferation and migration of pulmonary arterial smooth muscle cells (PASMCs), enhanced cellular functions that have been linked to increases in the cell membrane protein aquaporin 1 (AQP1). However, the mechanisms underlying the increased AQP1 abundance have not been fully elucidated. Here we present data that establishes a novel interaction between AQP1 and the proteolytic enzyme caspase-3. In silico analysis of the AQP1 protein reveals two caspase-3 cleavage sites on its C-terminal tail, proximal to known ubiquitin sites. Using biotin proximity ligase techniques, we establish that AQP1 and caspase-3 interact in both human embryonic kidney (HEK) 293A cells and rat PASMCs. Furthermore, we demonstrate that AQP1 levels increase and decrease with enhanced caspase-3 activity and inhibition, respectively. Ultimately, further work characterizing this interaction could provide the foundation for novel PH therapeutics.NEW & NOTEWORTHY Pulmonary arterial smooth muscle cells (PASMCs) are integral to pulmonary vascular remodeling, a characteristic of pulmonary arterial hypertension (PAH). PASMCs isolated from robust animal models of disease demonstrate enhanced proliferation and migration, pathological functions associated with increased abundance of the membrane protein aquaporin 1 (AQP1). We present evidence of a novel interaction between the proteolytic enzyme caspase-3 and AQP1, which may control AQP1 abundance. These data suggest a potential new target for novel PAH therapies.
Keywords: lung; myocytes; pulmonary vascular disease.
Conflict of interest statement
Larissa Shimoda is an editor of
Figures



Similar articles
-
Aquaporin 1 confers apoptosis resistance in pulmonary arterial smooth muscle cells from the SU5416 hypoxia rat model.Physiol Rep. 2024 Aug;12(16):e16156. doi: 10.14814/phy2.16156. Physiol Rep. 2024. PMID: 39175041 Free PMC article.
-
Hypoxia-induced migration in pulmonary arterial smooth muscle cells requires calcium-dependent upregulation of aquaporin 1.Am J Physiol Lung Cell Mol Physiol. 2012 Aug 15;303(4):L343-53. doi: 10.1152/ajplung.00130.2012. Epub 2012 Jun 8. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22683574 Free PMC article.
-
Aldosterone Contributed to Pulmonary Arterial Hypertension Development via Stimulating Aquaporin Expression and Pulmonary Arterial Smooth Muscle Cells Proliferation.Pharmacology. 2020;105(7-8):405-415. doi: 10.1159/000504228. Epub 2020 Jan 29. Pharmacology. 2020. PMID: 31995798
-
Aquaporin 1-mediated changes in pulmonary arterial smooth muscle cell migration and proliferation involve β-catenin.Am J Physiol Lung Cell Mol Physiol. 2017 Nov 1;313(5):L889-L898. doi: 10.1152/ajplung.00247.2016. Epub 2017 Aug 10. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28798257 Free PMC article.
-
Identification of Novel Therapeutic Targets for Pulmonary Arterial Hypertension.Int J Mol Sci. 2018 Dec 17;19(12):4081. doi: 10.3390/ijms19124081. Int J Mol Sci. 2018. PMID: 30562953 Free PMC article. Review.
References
-
- Yun X, Philip NM, Jiang H, Smith Z, Huetsch JC, Damarla M, Suresh K, Shimoda LA. Upregulation of aquaporin 1 mediates increased migration and proliferation in pulmonary vascular cells from the rat su5416/hypoxia model of pulmonary hypertension. Front Physiol 12: 763444, 2021.doi:10.3389/fphys.2021.763444. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

