Efficacy and tolerability of adjunctive lacosamide in patients aged <4 years with focal seizures
- PMID: 38375995
- PMCID: PMC10963285
- DOI: 10.1002/acn3.52004
Efficacy and tolerability of adjunctive lacosamide in patients aged <4 years with focal seizures
Abstract
Objective: Primary objective was to evaluate efficacy of lacosamide administered concomitantly with 1-3 antiseizure medications in young children with uncontrolled focal (partial-onset) seizures.
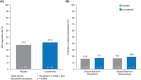
Methods: Double-blind, parallel-group trial (SP0967: NCT02477839/2013-000717-20) conducted between June 2015 and May 2020 at hospitals and clinics in 25 countries. Patients (aged ≥1 month to <4 years) with uncontrolled focal seizures were randomized 1:1 to adjunctive lacosamide or placebo using an interactive voice/web response system and stratified by age. After a 20-day titration period, patients who reached target-dose range (8-12 mg/kg/day) entered a 7-day maintenance period. Region-specific primary efficacy variables were based on ≤72-h video-electroencephalograms: change in average daily frequency (ADF) of electrographic focal seizures as measured on end-of-maintenance video-electroencephalogram versus end-of-baseline video-electroencephalogram (United States); 50% responder rate (≥50% reduction in ADF of focal seizures) during maintenance (European Union).
Results: In total, 255 patients were randomized (lacosamide/placebo: 128/127) and received ≥1 trial medication dose. Percentage reduction in ADF of focal seizures for lacosamide (116 patients) versus placebo (120 patients) was 3.2% (95% confidence interval = -13.6 to 17.5, p = 0.69). 50% responder rate was 41.4% for lacosamide (116 patients), 37.5% for placebo (120 patients) (p = 0.58). Treatment-emergent adverse events were reported by 44.5% of lacosamide-treated patients (placebo 51.2%).
Interpretation: Adjunctive lacosamide did not show superior efficacy versus placebo in young children with focal seizures. However, efficacy variables were potentially affected by high variability and low reliability between readers in video-electroencephalogram interpretation. Lacosamide was generally well tolerated; safety profile was acceptable and consistent with that in adults and children aged ≥4 years.
© 2024 UCB Biopharma SRL. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
I.M. and Y.‐T.N. have nothing to report. C.B., J.C., C.M., and H.M. are employees of UCB Pharma. A.B. was employed by UCB Pharma at the time of the trial, and is currently employed by Otsuka Pharmaceutical Development & Commercialization, Inc. C.B. and J.C. have received UCB Pharma stocks from their employment. A.B. owned UCB Pharma stocks during the conduct of the trial. C.M. and H.M. have received UCB Pharma stocks/stock options from their employment. M.K.F. has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from BioMarin, Nutricia Hungary, and PTC Therapeutics, and support for attending meetings and/or travel from BioMarin and PTC Therapeutics.
Figures



References
-
- Aaberg KM, Gunnes N, Bakken IJ, et al. Incidence and prevalence of childhood epilepsy: a nationwide cohort study. Pediatrics. 2017;139:e20163908. - PubMed
-
- Mudigoudar B, Weatherspoon S, Wheless JW. Emerging antiepileptic drugs for severe pediatric epilepsies. Semin Pediatr Neurol. 2016;23:167‐179. - PubMed
-
- UCB Inc . VIMPAT (Lacosamide) US Prescribing Information. 2023. [November 7, 2023]; https://www.ucb‐usa.com/vimpat‐prescribing‐information.pdf
-
- UCB Pharma SA . VIMPAT (Lacosamide) EU Summary of Product Characteristics. 2022. [November 8, 2023]; https://www.ema.europa.eu/en/documents/product‐information/vimpat‐epar‐p...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
