Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review
- PMID: 38376087
- PMCID: PMC10806356
- DOI: 10.1159/000531890
Low-Dose Oral Minoxidil for Alopecia: A Comprehensive Review
Abstract
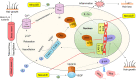
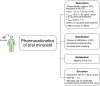
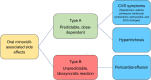
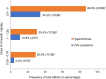
Low-dose oral minoxidil (LDOM) has demonstrated a promising safety and efficacy profile in the treatment of various hair disorders, including male androgenetic alopecia (AGA) and female-pattern hair loss (FPHL); however, it lacks FDA approval. The usual LDOM starting dose for male AGA is 1-5 mg/day, depending on physician preference and the patient's condition. For FPHL, it is 0.5-1 mg/day. The maximum dose is generally 5 mg/day. If patients respond well without major side effects, the dose may be gradually increased since the LDOM's efficacy appears to be dose-dependent. Patients may use LDOM long term if the treatment outcome is satisfactory. The common side effects of LDOM are hypertrichosis and cardiovascular symptoms. Females are more prone to hypertrichosis than males. The side effects of LDOM can be categorized as (a) dose-dependent type A side effects (hypertrichosis and cardiovascular symptoms) and (b) idiosyncratic type B side effects (pericardial effusion). Minoxidil acts via multiple pathways. Although minoxidil has a relatively short half-life of around 4 h, its hypotensive effect may last approximately 72 h. Effective treatments for alopecia are limited. Therefore, LDOM could be an important addition to the available therapies for managing some hair disorders, including AGA.
Keywords: Androgenetic alopecia; Female-pattern hair loss; Hair disorders; Minoxidil; Oral administration.
© 2023 S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Efficacy and safety of low-dose oral minoxidil in the management of androgenetic alopecia.Expert Opin Pharmacother. 2024 Feb;25(2):139-147. doi: 10.1080/14656566.2024.2314087. Epub 2024 Feb 6. Expert Opin Pharmacother. 2024. PMID: 38315101 Review.
-
Assessing low-dose oral minoxidil efficacy in androgenetic alopecia: a comparative study of AGA and AGA unmasked by telogen effluvium.Arch Dermatol Res. 2024 Aug 12;316(8):514. doi: 10.1007/s00403-024-03257-w. Arch Dermatol Res. 2024. PMID: 39133308
-
Low-dose oral minoxidil does not significantly affect blood pressure: A systematic review and meta-analysis.J Am Acad Dermatol. 2025 Mar;92(3):554-555. doi: 10.1016/j.jaad.2024.10.057. Epub 2024 Nov 7. J Am Acad Dermatol. 2025. PMID: 39521141
-
Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients.J Am Acad Dermatol. 2021 Jun;84(6):1644-1651. doi: 10.1016/j.jaad.2021.02.054. Epub 2021 Feb 24. J Am Acad Dermatol. 2021. PMID: 33639244
-
Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia.J Dermatolog Treat. 2022 Nov;33(7):2946-2962. doi: 10.1080/09546634.2022.2109567. Epub 2022 Aug 15. J Dermatolog Treat. 2022. PMID: 35920739 Review.
Cited by
-
Characterization and Management of Adverse Events of Low-Dose Oral Minoxidil Treatment for Alopecia: A Narrative Review.J Clin Med. 2025 Mar 7;14(6):1805. doi: 10.3390/jcm14061805. J Clin Med. 2025. PMID: 40142611 Free PMC article. Review.
-
Gender Differences in Adverse Effects and Dosing Practices of Low-Dose Oral Minoxidil for Androgenetic Alopecia: A Retrospective Analysis of 310 Patients.Skin Appendage Disord. 2025 Jun;11(3):227-231. doi: 10.1159/000542630. Epub 2024 Nov 25. Skin Appendage Disord. 2025. PMID: 40475096
-
Effectiveness of platelet-rich plasma in treating female hair loss: A systematic review and meta-analysis of randomized controlled trials.Skin Res Technol. 2024 Aug;30(8):e70004. doi: 10.1111/srt.70004. Skin Res Technol. 2024. PMID: 39177365 Free PMC article.
-
Fractional microporation-guided delivery of nanoencapsulated drugs for enhanced cutaneous and follicular absorption: a comparison of ablative laser and radiofrequency microneedling.Drug Deliv Transl Res. 2025 May 29. doi: 10.1007/s13346-025-01885-x. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40439860
-
Development of a sensor-compatible vascular microphysiological system for metabolic monitoring during drug-induced endothelial injury.Anal Sci. 2025 Jul 17. doi: 10.1007/s44211-025-00825-6. Online ahead of print. Anal Sci. 2025. PMID: 40676483
References
LinkOut - more resources
Full Text Sources
Miscellaneous

