Absence of CD80 reduces HSV-1 replication in the eye and delays reactivation but not latency levels
- PMID: 38376148
- PMCID: PMC10949485
- DOI: 10.1128/jvi.02010-23
Absence of CD80 reduces HSV-1 replication in the eye and delays reactivation but not latency levels
Abstract
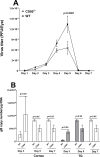
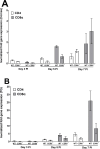
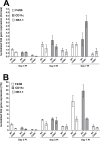
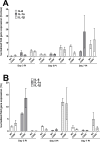
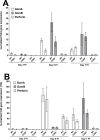
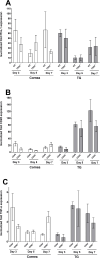
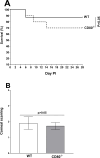
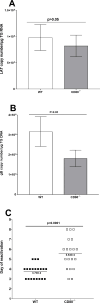
Herpes simplex virus-1 (HSV-1) infections are among the most frequent serious viral eye infections in the U.S. and are a major cause of viral-induced blindness. HSV-1 infection is known to induce T cell activation, proliferation, and differentiation that play crucial roles in the development of virus-induced inflammatory lesions, leading to eye disease and causing chronic corneal damage. CD80 is a co-stimulatory molecule and plays a leading role in T cell differentiation. Previous efforts to limit lesion severity by controlling inflammation at the cellular level led us to ask whether mice knocked out for CD80 would show attenuated virus replication following reactivation. By evaluating the effects of CD80 activity on primary and latent infection, we found that in the absence of CD80, virus replication in the eyes and virus reactivation in latent trigeminal ganglia were both significantly reduced. However, latency in latently infected CD80-/- mice did not differ significantly from that in wild-type (WT) control mice. Reduced virus replication in the eyes of CD80-/- mice correlated with significantly expanded CD11c gene expression as compared to WT mice. Taken together, our results indicate that suppression of CD80 could offer significant beneficial therapeutic effects in the treatment of Herpes Stromal Keratitis (HSK).IMPORTANCEOf the many problems associated with recurrent ocular infection, reducing virus reactivation should be a major goal of controlling ocular herpes simplex virus-1 (HSV-1) infection. In this study, we have shown that the absence of CD80 reduces HSV-1 reactivation, which marks the establishment of a previously undescribed mechanism underlying viral immune evasion that could be exploited to better manage HSV infection.
Keywords: CD8; PD-1; corneal scarring; latency; reactivation; virus replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Presence of CD80 and Absence of LAT in Modulating Cellular Infiltration and HSV-1 Latency.Viruses. 2024 Aug 29;16(9):1379. doi: 10.3390/v16091379. Viruses. 2024. PMID: 39339855 Free PMC article.
-
An M2 Rather than a TH2 Response Contributes to Better Protection against Latency Reactivation following Ocular Infection of Naive Mice with a Recombinant Herpes Simplex Virus 1 Expressing Murine Interleukin-4.J Virol. 2018 Apr 27;92(10):e00051-18. doi: 10.1128/JVI.00051-18. Print 2018 May 15. J Virol. 2018. PMID: 29491152 Free PMC article.
-
Absence of CD28-CTLA4-PD-L1 Costimulatory Molecules Reduces Herpes Simplex Virus 1 Reactivation.mBio. 2021 Aug 31;12(4):e0117621. doi: 10.1128/mBio.01176-21. Epub 2021 Jul 20. mBio. 2021. PMID: 34281386 Free PMC article.
-
[Battle with herpes for 37 years].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):145-66; discussion 167. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854108 Review. Japanese.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
Cited by
-
Presence of CD80 and Absence of LAT in Modulating Cellular Infiltration and HSV-1 Latency.Viruses. 2024 Aug 29;16(9):1379. doi: 10.3390/v16091379. Viruses. 2024. PMID: 39339855 Free PMC article.
-
Transcriptomic and Metabolomic Analyses Reveal the Attenuating Role of Cordycepin and Cordyceps militaris Extract on Acute Liver Injury Induced by LPS in Piglets.Animals (Basel). 2024 Oct 5;14(19):2873. doi: 10.3390/ani14192873. Animals (Basel). 2024. PMID: 39409822 Free PMC article.
-
A review of HSV pathogenesis, vaccine development, and advanced applications.Mol Biomed. 2024 Aug 29;5(1):35. doi: 10.1186/s43556-024-00199-7. Mol Biomed. 2024. PMID: 39207577 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

