A phase 1 study in healthy volunteers to investigate the safety, tolerability, and pharmacokinetics of VIR-2482: a monoclonal antibody for the prevention of severe influenza A illness
- PMID: 38376227
- PMCID: PMC10988998
- DOI: 10.1128/aac.01273-23
A phase 1 study in healthy volunteers to investigate the safety, tolerability, and pharmacokinetics of VIR-2482: a monoclonal antibody for the prevention of severe influenza A illness
Abstract
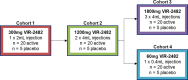
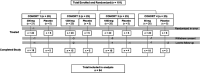
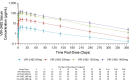
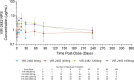
The objective of this study was to evaluate the safety, tolerability, pharmacokinetics (PK), and immunogenicity of VIR-2482 in healthy adult subjects. A phase 1, first-in-human, randomized, double-blind, placebo-controlled dose-escalation study was conducted. One hundred participants were allocated to four cohorts (60 mg, 300 mg, 1,200 mg, and 1,800 mg). In each cohort, participants were randomized in a 4:1 ratio (active:placebo) to receive either VIR-2482 or volume-matched placebo by gluteal intramuscular injection. Participants remained at the investigative site under observation for 48 h, and adverse events (AEs) were collected for 56 days. PK and immunogenicity were measured up to 52 weeks post-dose. VIR-2482 was well tolerated at all doses studied. The overall incidence of AEs was comparable between VIR-2482 (68.8%) and placebo (85.0%). Nineteen VIR-2482 (23.8%) and six placebo (30.0%) recipients had Grade 1 or 2 AEs that were considered to be related to the study intervention. There were no treatment-related serious AEs. Injection-site reactions (ISRs) were reported in six (7.5%) VIR-2482 recipients, while no such reactions were reported among the placebo recipients. All ISRs were Grade 1, and there was no relationship with the dose. Median VIR-2482 serum elimination half-life ranged from 56.7 to 70.6 days across cohorts. The serum area under the curve and Cmax were dose-proportional. Nasopharyngeal VIR-2482 concentrations were approximately 2%-5% of serum levels and were less than dose-proportional. The incidence of immunogenicity across all cohorts was 1.3%. Overall, the safety, tolerability, and pharmacokinetic profile of VIR-2482 at doses up to 1,800 mg supported its further investigation as a long-acting antibody for the prevention of influenza A illness. This study has been registered at ClinicalTrials.gov under identifier NCT04033406.
Keywords: VIR-2482; human immunoglobulin G1; influenza; monoclonal antibodies; prophylaxis.
Conflict of interest statement
The following authors are Vir employees and stockholders: D.P., J.E.S., M.A., M.C.F., A.P., M.A.S., D.C., E.M., K.B., W.W.Y., and M.R. P.G. has no financial disclosures to report.
Figures
References
-
- World Health Organization . 2023. Influenza (seasonal). Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Retrieved 15 Sep 2023.
-
- Centers for Disease Control and Prevention . 2023. Vaccine effectiveness studies. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/how-the.... Retrieved 15 Sep 2023.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

