A High-Quality Blue Whale Genome, Segmental Duplications, and Historical Demography
- PMID: 38376487
- PMCID: PMC10919930
- DOI: 10.1093/molbev/msae036
A High-Quality Blue Whale Genome, Segmental Duplications, and Historical Demography
Abstract
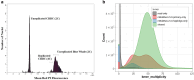
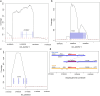
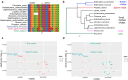
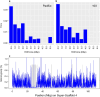
The blue whale, Balaenoptera musculus, is the largest animal known to have ever existed, making it an important case study in longevity and resistance to cancer. To further this and other blue whale-related research, we report a reference-quality, long-read-based genome assembly of this fascinating species. We assembled the genome from PacBio long reads and utilized Illumina/10×, optical maps, and Hi-C data for scaffolding, polishing, and manual curation. We also provided long read RNA-seq data to facilitate the annotation of the assembly by NCBI and Ensembl. Additionally, we annotated both haplotypes using TOGA and measured the genome size by flow cytometry. We then compared the blue whale genome with other cetaceans and artiodactyls, including vaquita (Phocoena sinus), the world's smallest cetacean, to investigate blue whale's unique biological traits. We found a dramatic amplification of several genes in the blue whale genome resulting from a recent burst in segmental duplications, though the possible connection between this amplification and giant body size requires further study. We also discovered sites in the insulin-like growth factor-1 gene correlated with body size in cetaceans. Finally, using our assembly to examine the heterozygosity and historical demography of Pacific and Atlantic blue whale populations, we found that the genomes of both populations are highly heterozygous and that their genetic isolation dates to the last interglacial period. Taken together, these results indicate how a high-quality, annotated blue whale genome will serve as an important resource for biology, evolution, and conservation research.
Keywords: animal genomes; body size; cetaceans; conservation; developmental biology; evolution; genetic diversity; segmental duplications.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Archer FI, Brownell RL Jr, Hancock-Hanser BL, Morin PA, Robertson KM, Sherman KK, Calambokidis J, Urbán R J, Rosel PE, Mizroch SA, et al. Revision of fin whale Balaenoptera physalus (Linnaeus, 1758) subspecies using genetics. J Mammal. 2019:100(5):1653–1670. 10.1093/jmammal/gyz121. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

