Role of zonisamide in advanced Parkinson's disease: a randomized placebo-controlled study
- PMID: 38376645
- PMCID: PMC10943138
- DOI: 10.1007/s10072-024-07396-w
Role of zonisamide in advanced Parkinson's disease: a randomized placebo-controlled study
Abstract
Background: Zonisamide (ZNS) has shown some efficacy in motor symptoms of PD; however, more evidence is lacking, and its effects on nonmotor symptoms (NMSs) and quality of life (QoL) remain to be investigated. This randomized double-blinded placebo-controlled crossover study investigated the effect of ZNS on motor and NMS symptoms and QoL in advanced PD.
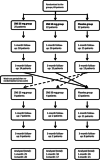
Methods: PD patients with Hoehn and Yahr stage ≥ 2 ("On" state) and at least 2 h off time daily were randomized to groups: ZNS 25 mg, ZNS 50 mg and placebo. Groups were assessed at baseline and at the 1- and 3-month follow-ups. The primary endpoint was the change in the total MDS-UPDRS III "On", while the secondary endpoint was the change in the total and parts I and IV MDS-UPDRS, Nonmotor Symptoms Scale and Parkinson's disease questionnaire-39 at the final assessment.
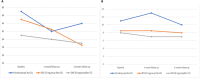
Results: Sixty-nine patients were assessed for efficacy at the 1-month follow-up, and 58 patients were assessed at the 3-month follow-up. The primary endpoint showed significant improvement in the ZNS 25 mg group compared to the placebo group (p = 0.009). At the final assessment, the ZNS 25 mg group showed significant improvement of total and part VI MDS-UPDRS, bradykinesia, tremor and functional impact of fluctuations compared to placebo. There was no change in dyskinesia, NMSs, QoL or side effects except for sedation.
Conclusion: ZNS has a favourable effect on motor symptoms in patients with wearing off as adjunctive therapy with other dopaminergic drugs, with no exacerbation of dyskinesia and a limited impact on NMSs and QoL.
Trial registration: Clinicaltrials.gov, NCT04182399, in 24/11/2019.
Keywords: Nonmotor symptoms; Parkinson̕ s disease; Quality of life; Wearing off; Zonisamide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Angelo Antonini has received compensation for consultancy and speaker-related activities from UCB, Britannia, AbbVie, Zambon, Bial, Ever Pharma, Theravance Biopharma, Roche, General Electric, Medscape. He receives research support from Chiesi Pharmaceuticals, Lundbeck, Bial, Movement Disorders Society, Horizon2020 Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081 and Cariparo Foundation. Other authors have no financial conflicts of interest to disclose, with no financial support or funding.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

