Functional cure is associated with younger age in children undergoing antiviral treatment for active chronic hepatitis B
- PMID: 38376650
- PMCID: PMC11014810
- DOI: 10.1007/s12072-023-10631-9
Functional cure is associated with younger age in children undergoing antiviral treatment for active chronic hepatitis B
Abstract
Background and aims: Functional cure is difficult to achieve using current antiviral therapies; moreover, limited data are available regarding treatment outcomes in children. This retrospective study aimed to assess the frequency of functional cure among children undergoing antiviral treatment for active chronic hepatitis B (CHB).
Methods: A total of 372 children aged 1-16 years, with active CHB were enrolled and underwent either nucleos(t)ide analog monotherapy or combination therapy with interferon-α (IFN-α) for 24-36 months. All children attended follow-up visits every 3 months. Functional cure was defined as evidence of hepatitis B virus (HBV) DNA loss, circulating hepatitis B e antigen (HBeAg) loss/seroconversion, and hepatitis B surface antigen (HBsAg) loss.
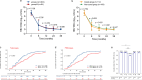
Results: After 36 months of antiviral treatment and/or follow-up visits, children with CHB aged 1- < 7 years exhibited higher rates of HBV DNA clearance, HBeAg seroconversion, and HBsAg loss than CHB children ≥ 7-16 years of age (93.75% versus [vs.] 86.21% [p < 0.0001]; 79.30% vs. 51.72% [p < 0.0001]; and 50.78% vs. 12.93% [p < 0.0001], respectively). Longitudinal investigation revealed more rapid dynamic reduction in HBV DNA, HBeAg, and HBsAg levels in children aged 1-7 years than in those aged ≥ 7-16 years with CHB. According to further age-stratified analysis, HBsAg loss rates were successively decreased in children with CHB who were 1- < 3, 3- < 7, 7- < 12, and 12-16 years of age (62.61% vs. 41.13% vs. 25.45% vs. 1.64%, respectively; p < 0.0001) at 36 months. In addition, baseline HBsAg level < 1,500 IU/mL was found to favor disease cure among these pediatric patients. No serious adverse events were observed throughout the study period.
Conclusion: Results of the present study demonstrated that children aged 1- < 7 years, with active CHB can achieve a high functional cure rate by undergoing antiviral therapy compared to those aged ≥ 7 years, who undergo antiviral therapy. These data support the use of antiviral treatment at an early age in children with CHB. However, future prospectively randomized controlled trials are necessary to validate the findings of this study.
Keywords: Antiviral therapy; Child; Chronic hepatitis B; Functional cure; HBV; HBV DNA loss; HBeAg seroconversion; HBsAg loss; Interferons; Nucleoside Analogs.
© 2024. The Author(s).
Conflict of interest statement
Min Zhang, Jing Li, Zhiqiang Xu, Peiyao Fan, Yi Dong, Fuchuan Wang, Yinjie Gao, Jianguo Yan, Lili Cao, Dong Ji, Danni Feng, Yanwei Zhong, Yang Zhang, Weiguo Hong, Chao Zhang and Fu-Sheng Wang have no conflicts of interest to disclose.
Figures


Similar articles
-
JNJ-73763989 and bersacapavir treatment in nucleos(t)ide analogue-suppressed patients with chronic hepatitis B: REEF-2.J Hepatol. 2024 Sep;81(3):404-414. doi: 10.1016/j.jhep.2024.03.046. Epub 2024 Apr 5. J Hepatol. 2024. PMID: 38583491 Clinical Trial.
-
Add-on pegylated interferon augments hepatitis B surface antigen clearance vs continuous nucleos(t)ide analog monotherapy in Chinese patients with chronic hepatitis B and hepatitis B surface antigen ≤ 1500 IU/mL: An observational study.World J Gastroenterol. 2020 Apr 7;26(13):1525-1539. doi: 10.3748/wjg.v26.i13.1525. World J Gastroenterol. 2020. PMID: 32308352 Free PMC article.
-
[A randomized controlled study on factors influencing the curative effect of sequential combined interferon and lamivudine therapy in children with immune-tolerant phase chronic hepatitis B].Zhonghua Gan Zang Bing Za Zhi. 2019 Aug 20;27(8):604-609. doi: 10.3760/cma.j.issn.1007-3418.2019.08.004. Zhonghua Gan Zang Bing Za Zhi. 2019. PMID: 31594077 Clinical Trial. Chinese.
-
Effect of combination treatment based on interferon and nucleos(t)ide analogues on functional cure of chronic hepatitis B: a systematic review and meta-analysis.Hepatol Int. 2020 Dec;14(6):958-972. doi: 10.1007/s12072-020-10099-x. Epub 2020 Nov 13. Hepatol Int. 2020. PMID: 33185803
-
Treatment of chronic hepatitis B: case selection and duration of therapy.J Gastroenterol Hepatol. 2002 Apr;17(4):409-14. doi: 10.1046/j.1440-1746.2002.02767.x. J Gastroenterol Hepatol. 2002. PMID: 11982721 Review.
Cited by
-
Functional cure induced by tenofovir alafenamide plus peginterferon-alpha-2b in young children with chronic hepatitis B: a case series study.BMC Infect Dis. 2024 Aug 15;24(1):830. doi: 10.1186/s12879-024-09723-0. BMC Infect Dis. 2024. PMID: 39148030 Free PMC article.
-
Virus-host interaction mechanisms in interferon therapy for hepatitis B virus infection: recent advances.Front Immunol. 2025 Jun 27;16:1603544. doi: 10.3389/fimmu.2025.1603544. eCollection 2025. Front Immunol. 2025. PMID: 40655152 Free PMC article. Review.
-
Steatosis and Interferon Associated with HBsAg Immune Control in Chronic Hepatitis B: A Real-World Propensity Score-Matched Study.Biomedicines. 2025 Jun 24;13(7):1538. doi: 10.3390/biomedicines13071538. Biomedicines. 2025. PMID: 40722612 Free PMC article.
-
Predictive value of HBeAg titer dynamics for HBsAg clearance in pediatric chronic hepatitis B.Front Pediatr. 2025 Apr 3;13:1539300. doi: 10.3389/fped.2025.1539300. eCollection 2025. Front Pediatr. 2025. PMID: 40248021 Free PMC article.
-
Update on the treatment navigation for functional cure of chronic hepatitis B: Expert consensus 2.0.Clin Mol Hepatol. 2025 Feb;31(Suppl):S134-S164. doi: 10.3350/cmh.2024.0780. Epub 2025 Jan 22. Clin Mol Hepatol. 2025. PMID: 39838828 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

