Robust and consistent measures of pattern separation based on information theory and demonstrated in the dentate gyrus
- PMID: 38377108
- PMCID: PMC10906873
- DOI: 10.1371/journal.pcbi.1010706
Robust and consistent measures of pattern separation based on information theory and demonstrated in the dentate gyrus
Abstract
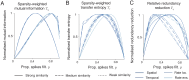
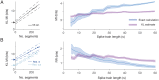
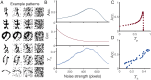
Pattern separation is a valuable computational function performed by neuronal circuits, such as the dentate gyrus, where dissimilarity between inputs is increased, reducing noise and increasing the storage capacity of downstream networks. Pattern separation is studied from both in vivo experimental and computational perspectives and, a number of different measures (such as orthogonalisation, decorrelation, or spike train distance) have been applied to quantify the process of pattern separation. However, these are known to give conclusions that can differ qualitatively depending on the choice of measure and the parameters used to calculate it. We here demonstrate that arbitrarily increasing sparsity, a noticeable feature of dentate granule cell firing and one that is believed to be key to pattern separation, typically leads to improved classical measures for pattern separation even, inappropriately, up to the point where almost all information about the inputs is lost. Standard measures therefore both cannot differentiate between pattern separation and pattern destruction, and give results that may depend on arbitrary parameter choices. We propose that techniques from information theory, in particular mutual information, transfer entropy, and redundancy, should be applied to penalise the potential for lost information (often due to increased sparsity) that is neglected by existing measures. We compare five commonly-used measures of pattern separation with three novel techniques based on information theory, showing that the latter can be applied in a principled way and provide a robust and reliable measure for comparing the pattern separation performance of different neurons and networks. We demonstrate our new measures on detailed compartmental models of individual dentate granule cells and a dentate microcircuit, and show how structural changes associated with epilepsy affect pattern separation performance. We also demonstrate how our measures of pattern separation can predict pattern completion accuracy. Overall, our measures solve a widely acknowledged problem in assessing the pattern separation of neural circuits such as the dentate gyrus, as well as the cerebellum and mushroom body. Finally we provide a publicly available toolbox allowing for easy analysis of pattern separation in spike train ensembles.
Copyright: © 2024 Bird et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Quantitative properties of a feedback circuit predict frequency-dependent pattern separation.Elife. 2020 Feb 20;9:e53148. doi: 10.7554/eLife.53148. Elife. 2020. PMID: 32077850 Free PMC article.
-
Corruption of the dentate gyrus by "dominant" granule cells: Implications for dentate gyrus function in health and disease.Neurobiol Learn Mem. 2016 Mar;129:69-82. doi: 10.1016/j.nlm.2015.09.005. Epub 2015 Sep 29. Neurobiol Learn Mem. 2016. PMID: 26391451 Free PMC article. Review.
-
Dendrites of dentate gyrus granule cells contribute to pattern separation by controlling sparsity.Hippocampus. 2017 Jan;27(1):89-110. doi: 10.1002/hipo.22675. Epub 2016 Nov 10. Hippocampus. 2017. PMID: 27784124 Free PMC article.
-
Kv4.1, a Key Ion Channel For Low Frequency Firing of Dentate Granule Cells, Is Crucial for Pattern Separation.J Neurosci. 2020 Mar 11;40(11):2200-2214. doi: 10.1523/JNEUROSCI.1541-19.2020. Epub 2020 Feb 11. J Neurosci. 2020. PMID: 32047055 Free PMC article.
-
Disambiguating the similar: the dentate gyrus and pattern separation.Behav Brain Res. 2012 Jan 1;226(1):56-65. doi: 10.1016/j.bbr.2011.08.039. Epub 2011 Sep 2. Behav Brain Res. 2012. PMID: 21907247 Review.
Cited by
-
Predictions enable top-down pattern separation in the macaque face-processing hierarchy.Nat Commun. 2024 Aug 21;15(1):7196. doi: 10.1038/s41467-024-51543-y. Nat Commun. 2024. PMID: 39169024 Free PMC article.
-
Phase information is conserved in sparse, synchronous population-rate-codes via phase-to-rate recoding.Nat Commun. 2023 Sep 30;14(1):6106. doi: 10.1038/s41467-023-41803-8. Nat Commun. 2023. PMID: 37777512 Free PMC article.
-
An Information-Geometric Formulation of Pattern Separation and Evaluation of Existing Indices.Entropy (Basel). 2024 Aug 29;26(9):737. doi: 10.3390/e26090737. Entropy (Basel). 2024. PMID: 39330071 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources

