Fluorescence-guided assessment of bone and soft-tissue sarcomas for predicting the efficacy of telomerase-specific oncolytic adenovirus
- PMID: 38377118
- PMCID: PMC10878518
- DOI: 10.1371/journal.pone.0298292
Fluorescence-guided assessment of bone and soft-tissue sarcomas for predicting the efficacy of telomerase-specific oncolytic adenovirus
Abstract
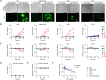
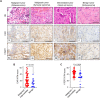
Bone and soft-tissue sarcomas are rare malignancies with histological diversity and tumor heterogeneity, leading to the lack of a common molecular target. Telomerase is a key enzyme for keeping the telomere length and human telomerase reverse transcriptase (hTERT) expression is often activated in most human cancers, including bone and soft-tissue sarcomas. For targeting of telomerase-positive tumor cells, we developed OBP-301, a telomerase-specific replication-competent oncolytic adenovirus, in which the hTERT promoter regulates adenoviral E1 gene for tumor-specific viral replication. In this study, we present the diagnostic potential of green fluorescent protein (GFP)-expressing oncolytic adenovirus OBP-401 for assessing virotherapy sensitivity using bone and soft-tissue sarcomas. OBP-401-mediated GFP expression was significantly associated with the therapeutic efficacy of OBP-401 in human bone and soft-tissue sarcomas. In the tumor specimens from 68 patients, malignant and intermediate tumors demonstrated significantly higher expression levels of coxsackie and adenovirus receptor (CAR) and hTERT than benign tumors. OBP-401-mediated GFP expression was significantly increased in malignant and intermediate tumors with high expression levels of CAR and hTERT between 24 and 48 h after infection. Our results suggest that the OBP-401-based GFP expression system is a useful tool for predicting the therapeutic efficacy of oncolytic virotherapy on bone and soft-tissue sarcomas.
Copyright: © 2024 Uotani et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Y.U. is the President and CEO of Oncolys BioPharma, Inc. H.T. and Tos.F. are consultants of Oncolys BioPharma, Inc. The other authors have no potential conflicts of interest to disclose.
Figures



Similar articles
-
Histone deacetylase inhibitor FR901228 enhances the antitumor effect of telomerase-specific replication-selective adenoviral agent OBP-301 in human lung cancer cells.Exp Cell Res. 2006 Feb 1;312(3):256-65. doi: 10.1016/j.yexcr.2005.10.026. Epub 2005 Dec 13. Exp Cell Res. 2006. PMID: 16356494
-
Bone and Soft-Tissue Sarcoma: A New Target for Telomerase-Specific Oncolytic Virotherapy.Cancers (Basel). 2020 Feb 18;12(2):478. doi: 10.3390/cancers12020478. Cancers (Basel). 2020. PMID: 32085583 Free PMC article. Review.
-
Real-Time Fluorescence Image-Guided Oncolytic Virotherapy for Precise Cancer Treatment.Int J Mol Sci. 2021 Jan 17;22(2):879. doi: 10.3390/ijms22020879. Int J Mol Sci. 2021. PMID: 33477279 Free PMC article. Review.
-
Preclinical evaluation of telomerase-specific oncolytic virotherapy for human bone and soft tissue sarcomas.Clin Cancer Res. 2011 Apr 1;17(7):1828-38. doi: 10.1158/1078-0432.CCR-10-2066. Epub 2011 Feb 16. Clin Cancer Res. 2011. PMID: 21325287
-
A simple detection system for adenovirus receptor expression using a telomerase-specific replication-competent adenovirus.Gene Ther. 2013 Jan;20(1):112-8. doi: 10.1038/gt.2011.213. Epub 2012 Jan 12. Gene Ther. 2013. PMID: 22241176
Cited by
-
Telomeres and telomerase in Sarcoma disease and therapy.Int J Med Sci. 2024 Aug 6;21(11):2065-2080. doi: 10.7150/ijms.97485. eCollection 2024. Int J Med Sci. 2024. PMID: 39239547 Free PMC article. Review.
-
Receptors and Host Factors for Enterovirus Infection: Implications for Cancer Therapy.Cancers (Basel). 2024 Sep 12;16(18):3139. doi: 10.3390/cancers16183139. Cancers (Basel). 2024. PMID: 39335111 Free PMC article. Review.
References
-
- Zhou Y, Yang D, Yang Q, Lv X, Huang W, Zhou Z, et al.. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat Commun. 2020;11(1):6322. Epub 2020/12/12. doi: 10.1038/s41467-020-20059-6 ; PubMed Central PMCID: PMC7730477. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

