The Immune Landscape of Pheochromocytoma and Paraganglioma: Current Advances and Perspectives
- PMID: 38377172
- PMCID: PMC11244254
- DOI: 10.1210/endrev/bnae005
The Immune Landscape of Pheochromocytoma and Paraganglioma: Current Advances and Perspectives
Erratum in
-
Correction to: "The Immune Landscape of Pheochromocytoma and Paraganglioma: Current Advances and Perspectives".Endocr Rev. 2025 Aug 8:bnaf024. doi: 10.1210/endrev/bnaf024. Online ahead of print. Endocr Rev. 2025. PMID: 40795353 No abstract available.
Abstract
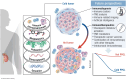
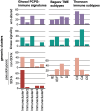
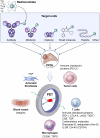
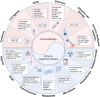
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors derived from neural crest cells from adrenal medullary chromaffin tissues and extra-adrenal paraganglia, respectively. Although the current treatment for PPGLs is surgery, optimal treatment options for advanced and metastatic cases have been limited. Hence, understanding the role of the immune system in PPGL tumorigenesis can provide essential knowledge for the development of better therapeutic and tumor management strategies, especially for those with advanced and metastatic PPGLs. The first part of this review outlines the fundamental principles of the immune system and tumor microenvironment, and their role in cancer immunoediting, particularly emphasizing PPGLs. We focus on how the unique pathophysiology of PPGLs, such as their high molecular, biochemical, and imaging heterogeneity and production of several oncometabolites, creates a tumor-specific microenvironment and immunologically "cold" tumors. Thereafter, we discuss recently published studies related to the reclustering of PPGLs based on their immune signature. The second part of this review discusses future perspectives in PPGL management, including immunodiagnostic and promising immunotherapeutic approaches for converting "cold" tumors into immunologically active or "hot" tumors known for their better immunotherapy response and patient outcomes. Special emphasis is placed on potent immune-related imaging strategies and immune signatures that could be used for the reclassification, prognostication, and management of these tumors to improve patient care and prognosis. Furthermore, we introduce currently available immunotherapies and their possible combinations with other available therapies as an emerging treatment for PPGLs that targets hostile tumor environments.
Keywords: cancer immunotherapy; immune system; neuroendocrine tumors; paraganglioma; perspectives; pheochromocytoma.
Published by Oxford University Press on behalf of the Endocrine Society 2024.
Figures


Similar articles
-
Pheochromocytoma and Paraganglioma.2025 Jul 21. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. 2025 Jul 21. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. PMID: 29465938 Free Books & Documents. Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma.Eur J Endocrinol. 2016 May;174(5):G1-G10. doi: 10.1530/EJE-16-0033. Eur J Endocrinol. 2016. PMID: 27048283
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
Cited by
-
Pheochromocytoma and Behçet's Disease: Exploring a Rare Coexistence.Cureus. 2025 May 26;17(5):e84812. doi: 10.7759/cureus.84812. eCollection 2025 May. Cureus. 2025. PMID: 40568255 Free PMC article.
-
Role of B cells in intratumoral MBTA immunotherapy of murine pheochromocytoma model.Best Pract Res Clin Endocrinol Metab. 2025 Jan;39(1):101941. doi: 10.1016/j.beem.2024.101941. Epub 2024 Sep 11. Best Pract Res Clin Endocrinol Metab. 2025. PMID: 39278811 Review.
-
A blood-based liquid biopsy analyzing soluble immune checkpoints and cytokines identifies distinct neuroendocrine tumors.J Exp Clin Cancer Res. 2025 Mar 5;44(1):82. doi: 10.1186/s13046-025-03337-3. J Exp Clin Cancer Res. 2025. PMID: 40038821 Free PMC article.
References
-
- Mete O, Asa SL, Gill AJ, Kimura N, de Krijger RR, Tischler A. Overview of the 2022 WHO classification of paragangliomas and pheochromocytomas. Endocr Pathol. 2022;33(1):90‐114. - PubMed
-
- Navalkissoor S, Gnanasegaran G, Grossman A. Optimisation of radioligand therapy in neuroendocrine tumours: current and evolving evidence. J Neuroendocrinol. 2022;34(11):e13208. - PubMed
-
- Jimenez C, Xu G, Varghese J, Graham PH, Campbell MT, Lu Y. New directions in treatment of metastatic or advanced pheochromocytomas and sympathetic paragangliomas: an American, contemporary, pragmatic approach. Curr Oncol Rep. 2022;24(1):89‐98. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

