Use of cancer-directed therapy at the end of life among adolescents and young adults
- PMID: 38377408
- PMCID: PMC11223859
- DOI: 10.1093/jnci/djae038
Use of cancer-directed therapy at the end of life among adolescents and young adults
Abstract
Background: Adolescents and young adults frequently receive chemotherapy near death. We know less about the use of targeted agents and immunotherapy or trends over time.
Methods: We conducted a retrospective cohort study of 1836 adolescents and young adults with cancer who died between 2009 and 2019 after receiving care at 1 of 3 sites (Dana-Farber Cancer Institute, Kaiser Permanente Northern California, and Kaiser Permanente Southern California). We reviewed electronic health data and medical records to examine use of cancer-directed therapy in the last 90 days of life, including chemotherapy, targeted therapy, immunotherapy, and investigational drugs.
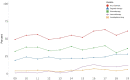
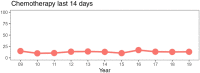
Results: Over the study period, 35% of adolescents and young adults received chemotherapy in the last 90 days of life; 24% received targeted therapy, 7% immunotherapy, and 5% investigational drugs. Additionally, 56% received at least 1 form of systemic cancer-directed therapy in the last 90 days of life. After adjustment for patient sex, race, ethnicity, age, site of care, diagnosis, and years from diagnosis to death, the proportion of adolescents and young adults receiving targeted therapy (odds ratio [OR] = 1.05 per year of death, 95% confidence interval [CI] = 1.02 to 1.10; P = .006), immunotherapy (OR = 1.27, 95% CI = 1.18 to 1.38; P < .0001), and any cancer-directed therapy (OR = 1.04, 95% CI = 1.01 to 1.08; P = .01) in the last 90 days of life increased over time.
Conclusions: More than half of adolescents and young adults receive cancer therapy in the last 90 days of life, and use of novel agents such as targeted therapy and immunotherapy is increasing over time. Although some adolescents and young adults may wish to continue cancer therapy while living with advanced disease, efforts are needed to ensure that use of cancer-directed therapy meets preferences of adolescents and young adults approaching death.
© The Author(s) 2024. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous