Serum aryl hydrocarbon receptor activity is associated with survival in patients with alcohol-associated hepatitis
- PMID: 38377466
- PMCID: PMC11268475
- DOI: 10.1097/HEP.0000000000000777
Serum aryl hydrocarbon receptor activity is associated with survival in patients with alcohol-associated hepatitis
Abstract
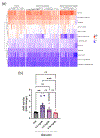
Background and aims: Patients with alcohol-associated hepatitis (AH) have an altered fecal metabolome, including reduced microbiota-derived tryptophan metabolites, which function as ligands for aryl hydrocarbon receptor (AhR). The aim of this study was to assess serum AhR ligand activity in patients with AH.
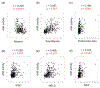
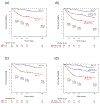
Approach and results: The study included 74 controls without AUD, 97 patients with AUD, and 330 patients with AH from 2 different multicenter cohorts (InTeam: 134, AlcHepNet: 196). Serum AhR activity was evaluated using an AhR reporter assay with HepG2-Lucia cells incubated with serum for 24 hours. Serum AhR activity was significantly higher in patients with AH compared with both controls (1.59 vs. 0.96-fold change, p < 0.001) and patients with AUD (1.59 vs. 0.93, p < 0.001). In both AH cohorts, patients with AhR activity ≥ 2.09 had significantly lower cumulative survival rates at 30, 60, 90, and 180 days compared to those with AhR activity < 2.09. When serum AhR activity was used to further stratify patients with severe AH, the cumulative 30, 60, 90, and 180-day survival rates for patients with severe AH and the AhR activity ≥ 2.09 group were all significantly lower than those with an AhR activity < 2.09 group.
Conclusions: Serum AhR activity was significantly higher in patients with AH compared with controls and individuals with AUD, and this increased activity was associated with higher mortality. Consequently, serum AhR activity holds potential as a prognostic marker.
Copyright © 2024 American Association for the Study of Liver Diseases.
Conflict of interest statement
B.S. has been consulting for Ferring Research Institute, HOST Therabiomics, Intercept Pharmaceuticals, Mabwell Therapeutics, Patara Pharmaceuticals, Surrozen and Takeda. B.S.’s institution UC San Diego has received research support from Axial Biotherapeutics, BiomX, ChromoLogic, CymaBay Therapeutics, NGM Biopharmaceuticals, Prodigy Biotech and Synlogic Operating Company. B.S. is founder of Nterica Bio. UC San Diego has filed several patents with B.S. as inventor related to this work. D.L.S. has consulted for EnteroBiotix and delivered paid lectures for Norgine. J.G.A. received grants from Cook and Gilead (paid to the University of Alberta) and received consulting fees from Boehringer Ingelheim, AstraZeneca, Advanz and 89Bio. V.V. served on Ipsen Pharma: advisory board, and has travel grant from Intercept Pharmaceuticals.
Figures


References
-
- Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 2020;71:306–333. - PubMed
-
- Bataller R, Arab JP, Shah VH. Alcohol-Associated Hepatitis. N Engl J Med 2022;387:2436–2448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR001863/TR/NCATS NIH HHS/United States
- P30 DK120515/DK/NIDDK NIH HHS/United States
- R01 AA024726/AA/NIAAA NIH HHS/United States
- T32 DK007202/DK/NIDDK NIH HHS/United States
- P50 AA011999/AA/NIAAA NIH HHS/United States
- K99 AA031328/AA/NIAAA NIH HHS/United States
- R01 AA020703/AA/NIAAA NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- I01 BX004594/BX/BLRD VA/United States
- R37 AA020703/AA/NIAAA NIH HHS/United States
- KL2 TR001444/TR/NCATS NIH HHS/United States
- U01 AA026972/AA/NIAAA NIH HHS/United States
- U01 AA026939/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources

