LNK/SH2B3 loss of function increases susceptibility to murine and human atrial fibrillation
- PMID: 38377486
- PMCID: PMC11218690
- DOI: 10.1093/cvr/cvae036
LNK/SH2B3 loss of function increases susceptibility to murine and human atrial fibrillation
Abstract
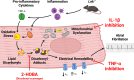
Aims: The lymphocyte adaptor protein (LNK) is a negative regulator of cytokine and growth factor signalling. The rs3184504 variant in SH2B3 reduces LNK function and is linked to cardiovascular, inflammatory, and haematologic disorders, including stroke. In mice, deletion of Lnk causes inflammation and oxidative stress. We hypothesized that Lnk-/- mice are susceptible to atrial fibrillation (AF) and that rs3184504 is associated with AF and AF-related stroke in humans. During inflammation, reactive lipid dicarbonyls are the major components of oxidative injury, and we further hypothesized that these mediators are critical drivers of the AF substrate in Lnk-/- mice.
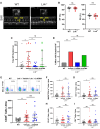
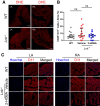
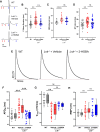
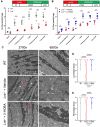
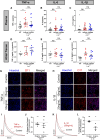
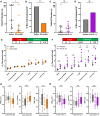
Methods and results: Lnk-/- or wild-type (WT) mice were treated with vehicle or 2-hydroxybenzylamine (2-HOBA), a dicarbonyl scavenger, for 3 months. Compared with WT, Lnk-/- mice displayed increased AF duration that was prevented by 2-HOBA. In the Lnk-/- atria, action potentials were prolonged with reduced transient outward K+ current, increased late Na+ current, and reduced peak Na+ current, pro-arrhythmic effects that were inhibited by 2-HOBA. Mitochondrial dysfunction, especially for Complex I, was evident in Lnk-/- atria, while scavenging lipid dicarbonyls prevented this abnormality. Tumour necrosis factor-α (TNF-α) and interleukin-1 beta (IL-1β) were elevated in Lnk-/- plasma and atrial tissue, respectively, both of which caused electrical and bioenergetic remodelling in vitro. Inhibition of soluble TNF-α prevented electrical remodelling and AF susceptibility, while IL-1β inhibition improved mitochondrial respiration but had no effect on AF susceptibility. In a large database of genotyped patients, rs3184504 was associated with AF, as well as AF-related stroke.
Conclusion: These findings identify a novel role for LNK in the pathophysiology of AF in both experimental mice and humans. Moreover, reactive lipid dicarbonyls are critical to the inflammatory AF substrate in Lnk-/- mice and mediate the pro-arrhythmic effects of pro-inflammatory cytokines, primarily through electrical remodelling.
Keywords: Atrial fibrillation; Inflammation; Isolevuglandins; Oxidative stress; Pro-inflammatory cytokines.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: K.T.M. has a pending patent application, and A.K. is a co-inventor on US Patent # 14/232,615, both with Metabolic Technologies, Inc. and Vanderbilt University.
Figures

Comment in
-
A mechanistic LNK between inflammation and atrial fibrillation?Cardiovasc Res. 2024 Jul 2;120(8):814-816. doi: 10.1093/cvr/cvae083. Cardiovasc Res. 2024. PMID: 38713542 Free PMC article. No abstract available.
References
-
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:E199–E267. - PMC - PubMed
-
- Shoemaker MB, Hemnes AR, Robbins IM, Langberg JJ, Ellis CR, Aznaurov SG, Fredi JL, Slosky DA, Roden DM, Murray KT, Piana RN, Mendes LA, Whalen SP. Left atrial hypertension after repeated catheter ablations for atrial fibrillation. J Am Coll Cardiol 2011;57:1918–1919. - PubMed
-
- Devalliere J, Charreau B. The adaptor Lnk (SH2B3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem Pharmacol 2011;82:1391–1402. - PubMed
-
- Alexander MR, Hank S, Dale BL, Himmel L, Zhong X, Smart CD, Fehrenbach DJ, Chen YH, Prabakaran N, Tirado B, Centrella M, Ao MF, Du LP, Shyr Y, Levy D, Madhur MS. A single nucleotide polymorphism in SH2B3/LNK promotes hypertension development and renal damage. Circ Res 2022;131:731–747. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- TR000445/National Center for Advancing Translational Sciences of the National Institute of Health
- R01 HL147818/HL/NHLBI NIH HHS/United States
- R01 HL096844/HL/NHLBI NIH HHS/United States
- R03 HL155041/HL/NHLBI NIH HHS/United States
- R01 DK056942/DK/NIDDK NIH HHS/United States
- 18SFRN34230125/American Heart Association
- Vanderbilt Cell Imaging Shared Resource
- R35 HL150783/HL/NHLBI NIH HHS/United States
- HL096844/NH/NIH HHS/United States
- R01 HL144941/HL/NHLBI NIH HHS/United States
- R01 HL133127/HL/NHLBI NIH HHS/United States
- Vanderbilt Translational Pathology Shared Resource
- IK2 BX005828/BX/BLRD VA/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- S10 OD021630/OD/NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

