Single-agent metronomic versus weekly oral vinorelbine as first-line chemotherapy in patients with HR-positive/HER2-negative advanced breast cancer: The randomized Tempo Breast study
- PMID: 38377732
- PMCID: PMC10891320
- DOI: 10.1016/j.breast.2024.103681
Single-agent metronomic versus weekly oral vinorelbine as first-line chemotherapy in patients with HR-positive/HER2-negative advanced breast cancer: The randomized Tempo Breast study
Abstract
Introduction: Single-agent oral vinorelbine is a standard of care for hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer (ABC) that has progressed on endocrine therapy. Metronomic administration may offer a better balance of efficacy and safety than standard regimens, but data from previous trials are scarce.
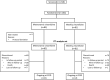
Methods: In this open-label, multicenter, phase II trial, patients were randomized to oral vinorelbine administered on a metronomic (50 mg three times weekly) or weekly (60 mg/m2 in cycle 1, increasing to 80 mg/m2 if well tolerated) schedule. Treatment was continued until disease progression or intolerance. The primary endpoint was disease control rate (DCR, the proportion of patients with a best overall confirmed response of CR, PR, or stable disease lasting 6 months or more).
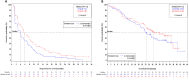
Results: One-hundred sixty-three patients were randomized and treated. The DCR was 63.4% (95% confidence interval [CI]: 52.0-73.8) with metronomic vinorelbine and 72.8% (95% CI: 61.8-82.1) with weekly vinorelbine. Weekly vinorelbine was also associated with longer progression-free survival (5.6 vs 4.0 months) and overall survival (26.7 vs 22.3 months) than metronomic vinorelbine, but was associated with more adverse events.
Conclusions: In this randomized phase II trial, single-agent metronomic oral vinorelbine was effective and well tolerated as first-line chemotherapy for patients with HR-positive/HER2-negative ABC. Formal comparisons are not done in this phase II study and one can simply observe that confidence intervals of all endpoints overlap. When deciding for a chemotherapy after failure of endocrine therapy and CDK 4/6 inhibitors, oral vinorelbine might be an option to be given with either schedule.
Clinical trial registration number: EudraCT 2014-003860-19.
Keywords: Administration; Chemotherapy; Disease control rate; HR+/HER2-advanced breast cancer; Metronomic; Oral; Vinorelbine; Weekly administration.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declarations of competing interest GF: scientific coordinator and principal investigator of the study; participated in advisory boards, acted as a speaker, and conducted training sessions on behalf of Pierre Fabre. NM-J: received consultancy fees from AstraZeneca, Daiichi Sankyo, Pfizer, Novartis, Lilly, Pierre Fabre, Roche, and GSK; and travel support from Roche, AstraZeneca, Pfizer, and Novartis. MM: received personal fees from Roche, Novartis, and Eisai; and other financial support from Roche and Lilly. LC: received honoraria from AstraZeneca, MSD, and Pfizer; Novartis, and Gilead. MP: received consultancy fees from AstraZeneca, Novartis, Pfizer, Eli Lilly, and Amgen. EP: received consultancy fees from AstraZeneca, Daiichi Sankyo, Pfizer, Novartis, Lilly, Pierre Fabre, Roche, and GSK; research funding from Daiichi Sankyo, Pfizer, Lilly, Novartis, Seattle Genetics, AstraZeneca, and Roche; and travel support from AstraZeneca, GSK, Pfizer, Lilly, and GSK. JE: received consultancy fees from AstraZeneca, Daiichi Sankyo, Pfizer, Novartis, Lilly, Pierre Fabre, Roche, and Tesaro; research funding from Daiichi Sankyo, Pfizer, Lilly, Novartis, Seattle Genetics, AstraZeneca, Roche, and Odonate; and travel support from AstraZeneca, Daiichi Sankyo, Celgene, Pfizer, Novartis, Lilly, and Tesaro. AR: received honoraria for participating in advisory boards for Boehringer Ingelheim, Bristol Myers Squibb, and MSD; and in internal training events at Pfizer and AstraZeneca. MEC: participated in an advisory board for Eli Lilly and Pierre Fabre. CdA, GV, RR and CTTM: employees of Pierre Fabre. BK-B, MU, HB, SM, JBC, TP, and NC: no conflicts to declare.
Figures
References
-
- Aapro M., Ruiz-Borrego M., Hegg R., Kukielka-Budny B., Morales S., Cinieri S., et al. Randomized phase II study evaluating weekly oral vinorelbine versus weekly paclitaxel in estrogen receptor-positive, HER2-negative patients with advanced breast cancer (NorBreast-231 trial) Breast. 2019;45:7–14. doi: 10.1016/j.breast.2019.01.009. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

