Stem cell migration drives lung repair in living mice
- PMID: 38377991
- PMCID: PMC11003834
- DOI: 10.1016/j.devcel.2024.02.003
Stem cell migration drives lung repair in living mice
Abstract
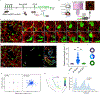
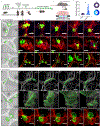
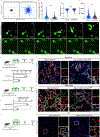
Tissue repair requires a highly coordinated cellular response to injury. In the lung, alveolar type 2 cells (AT2s) act as stem cells to replenish both themselves and alveolar type 1 cells (AT1s); however, the complex orchestration of stem cell activity after injury is poorly understood. Here, we establish longitudinal imaging of AT2s in murine intact tissues ex vivo and in vivo in order to track their dynamic behavior over time. We discover that a large fraction of AT2s become motile following injury and provide direct evidence for their migration between alveolar units. High-resolution morphokinetic mapping of AT2s further uncovers the emergence of distinct motile phenotypes. Inhibition of AT2 migration via genetic depletion of ArpC3 leads to impaired regeneration of AT2s and AT1s in vivo. Together, our results establish a requirement for stem cell migration between alveolar units and identify properties of stem cell motility at high cellular resolution.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests In the last 3 years, N.K. served as a consultant to Biogen Idec, Boehringer Ingelheim, Third Rock, Pliant, Samumed, LifeMax, Three Lake Partners, Optikira, Astra Zeneca, RohBar, Veracyte, Augmanity, CSL Behring, Galapagos, Sofinnova, and Thyron; reports equity in Pliant and Thyron; reports grants from Veracyte, Boehringer Ingelheim, and BMS; and reports non-financial support from MiRagen and Astra Zeneca. N.K. has IP on novel biomarkers and therapeutics in IPF licensed to Biotech and is a scientific founder of Thyron. P.R.T. serves as acting CEO of Iolux Inc. and serves as a consultant for Surrozen Inc., Cellarity Inc., and Celldom Inc. on work not related to the contents of this manuscript. P.R.T. received research funding support from United Therapeutics Inc, Ono Pharmaceuticals, and Boehringer Ingelheim.
Figures

References
Publication types
MeSH terms
Grants and funding
- R01 HL141852/HL/NHLBI NIH HHS/United States
- R01 HL160939/HL/NHLBI NIH HHS/United States
- R01 HL153375/HL/NHLBI NIH HHS/United States
- R01 HL127349/HL/NHLBI NIH HHS/United States
- P30 CA072720/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- F30 HL143911/HL/NHLBI NIH HHS/United States
- T32 CA200561/CA/NCI NIH HHS/United States
- R21 HL161556/HL/NHLBI NIH HHS/United States
- U01 HL145567/HL/NHLBI NIH HHS/United States
- U01 HL122626/HL/NHLBI NIH HHS/United States
- R01 HL155948/HL/NHLBI NIH HHS/United States
- R01 HL162629/HL/NHLBI NIH HHS/United States
- R01 HL146557/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

