The gut microbiome regulates the clinical efficacy of sulfasalazine therapy for IBD-associated spondyloarthritis
- PMID: 38378002
- PMCID: PMC10982976
- DOI: 10.1016/j.xcrm.2024.101431
The gut microbiome regulates the clinical efficacy of sulfasalazine therapy for IBD-associated spondyloarthritis
Abstract
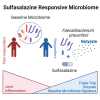
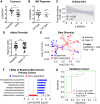
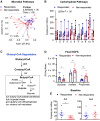
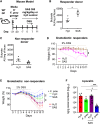
Sulfasalazine is a prodrug known to be effective for the treatment of inflammatory bowel disease (IBD)-associated peripheral spondyloarthritis (pSpA), but the mechanistic role for the gut microbiome in regulating its clinical efficacy is not well understood. Here, treatment of 22 IBD-pSpA subjects with sulfasalazine identifies clinical responders with a gut microbiome enriched in Faecalibacterium prausnitzii and the capacity for butyrate production. Sulfapyridine promotes butyrate production and transcription of the butyrate synthesis gene but in F. prausnitzii in vitro, which is suppressed by excess folate. Sulfasalazine therapy enhances fecal butyrate production and limits colitis in wild-type and gnotobiotic mice colonized with responder, but not non-responder, microbiomes. F. prausnitzii is sufficient to restore sulfasalazine protection from colitis in gnotobiotic mice colonized with non-responder microbiomes. These findings reveal a mechanistic link between the efficacy of sulfasalazine therapy and the gut microbiome with the potential to guide diagnostic and therapeutic approaches for IBD-pSpA.
Keywords: Faecalibacterium prausnitzii; butyrate; folate; inflammatory bowel disease; microbiome; spondyloarthritis; sulfasalazine.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Ossum A.M., Palm Ø., Lunder A.K., Cvancarova M., Banitalebi H., Negård A., Høie O., Henriksen M., Moum B.A., Høivik M.L., IBSEN Study Group Ankylosing Spondylitis and Axial Spondyloarthritis in Patients With Long-term Inflammatory Bowel Disease: Results From 20 Years of Follow-up in the IBSEN Study. J. Crohns Colitis. 2018;12:96–104. doi: 10.1093/ecco-jcc/jjx126. PubMed PMID: 28961700. - DOI - PubMed
-
- van Erp S.J., Brakenhoff L.K., van Gaalen F.A., van den Berg R., Fidder H.H., Verspaget H.W., Huizinga T.W., Veenendaal R.A., Wolterbeek R., van der Heijde D., et al. Classifying Back Pain and Peripheral Joint Complaints in Inflammatory Bowel Disease Patients: A Prospective Longitudinal Follow-up Study. J. Crohns Colitis. 2016;10:166–175. doi: 10.1093/ecco-jcc/jjv195. PubMed PMID: 26512134. - DOI - PubMed
-
- Palm Ø., Moum B., Jahnsen J., Gran J.T. The prevalence and incidence of peripheral arthritis in patients with inflammatory bowel disease, a prospective population-based study (the IBSEN study) Rheumatology. 2001;40:1256–1261. doi: 10.1093/rheumatology/40.11.1256. PubMed PMID: 11709609. - DOI - PubMed
-
- Ditisheim S., Fournier N., Juillerat P., Pittet V., Michetti P., Gabay C., Finckh A., Swiss IBD Cohort Study Group Inflammatory Articular Disease in Patients with Inflammatory Bowel Disease: Result of the Swiss IBD Cohort Study. Inflamm. Bowel Dis. 2015;21:2598–2604. doi: 10.1097/MIB.0000000000000548. PubMed PMID: 26244648. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

