Baculovirus entry into the central nervous system of Spodoptera exigua caterpillars is independent of the viral protein tyrosine phosphatase
- PMID: 38378139
- PMCID: PMC10878822
- DOI: 10.1098/rsob.230278
Baculovirus entry into the central nervous system of Spodoptera exigua caterpillars is independent of the viral protein tyrosine phosphatase
Abstract
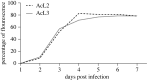
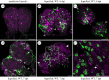
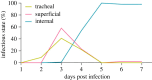
Neuroparasitism concerns the hostile take-over of a host's nervous system by a foreign invader, in order to alter the behaviour of the host in favour of the parasite. One of the most remarkable cases of parasite-induced host behavioural manipulation comprises the changes baculoviruses induce in their caterpillar hosts. Baculoviruses may manipulate caterpillar behaviour in two ways: hyperactivity (increased movement in the horizontal plane) and/or tree-top disease (movement to elevated levels in the vertical plane). Those behavioural changes are followed by liquefaction and death of the caterpillar. In Autographa californica multiple nucleopolyhedrovirus (AcMNPV)-infected Spodoptera exigua caterpillars, an enzymatic active form of the virally encoded protein tyrosine phosphatase (PTP) is needed for the expression of hyperactivity from 3 days post infection (dpi). Using eGFP-expressing recombinant AcMNPV strains, we show that infection of the caterpillar's central nervous system (CNS) can be observed primarily from 3 dpi onwards. In addition, we demonstrate that the structural and enzymatic function of PTP does not play a role in infection of the CNS. Instead we show that the virus entered the CNS via the trachea, progressing caudally to frontally through the CNS and that the infection progressed from the outermost cell layers towards the inner cell layers of the CNS, in a PTP independent manner. These findings help to further understand parasitic manipulation and the mechanisms by which neuroparasites infect the host nervous system to manipulate host behaviour.
Keywords: Autographa californica multiple nucleopolyhedrovirus; Spodoptera exigua; central nervous system; neuroparasitology; parasite-induced behavioural manipulation; protein tyrosine phosphatase.
Conflict of interest statement
We declare we have no competing interests.
Figures

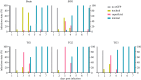
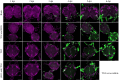
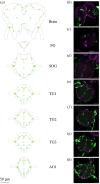

References
-
- Helluy S. 1984. Relations hôtes–parasite du trématode Microphallus papillorobustus (Rankin 1940). III. Facteurs impliqués dans les modifications du comportement des Gammarus hôtes intermédiaires et tests de prédation. Ann. Parasitol. Hum. Comp. 59, 41-56. (10.1051/parasite/1983581001) - DOI - PubMed
-
- Lafferty KD, Morris AK. 1996. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77, 1390-1397. (10.2307/2265536) - DOI
-
- Thomas F, Schmidt-Rhaesa A, Martin G, Manu C, Durand P, Renaud F. 2002. Do hairworms (Nematomorpha) manipulate the water seeking behavior of their terrestrial hosts? J. Evol. Biol. 15, 356-361. (10.1046/j.1420-9101.2002.00410.x) - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
