Milk fat globule-epidermal growth factor 8 (MFGE8) prevents intestinal fibrosis
- PMID: 38378253
- PMCID: PMC11248270
- DOI: 10.1136/gutjnl-2022-328608
Milk fat globule-epidermal growth factor 8 (MFGE8) prevents intestinal fibrosis
Abstract
Objective: Intestinal fibrosis is considered an inevitable consequence of chronic IBD, leading to stricture formation and need for surgery. During the process of fibrogenesis, extracellular matrix (ECM) components critically regulate the function of mesenchymal cells. We characterised the composition and function of ECM in fibrostenosing Crohn's disease (CD) and control tissues.
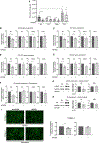
Design: Decellularised full-thickness intestinal tissue platforms were tested using three different protocols, and ECM composition in different tissue phenotypes was explored by proteomics and validated by quantitative PCR (qPCR) and immunohistochemistry. Primary human intestinal myofibroblasts (HIMFs) treated with milk fat globule-epidermal growth factor 8 (MFGE8) were evaluated regarding the mechanism of their antifibrotic response, and the action of MFGE8 was tested in two experimental intestinal fibrosis models.
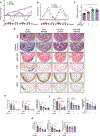
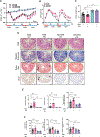
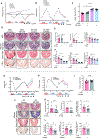
Results: We established and validated an optimal decellularisation protocol for intestinal IBD tissues. Matrisome analysis revealed elevated MFGE8 expression in CD strictured (CDs) tissue, which was confirmed at the mRNA and protein levels. Treatment with MFGE8 inhibited ECM production in normal control HIMF but not CDs HIMF. Next-generation sequencing uncovered functionally relevant integrin-mediated signalling pathways, and blockade of integrin αvβ5 and focal adhesion kinase rendered HIMF non-responsive to MFGE8. MFGE8 prevented and reversed experimental intestinal fibrosis in vitro and in vivo.
Conclusion: MFGE8 displays antifibrotic effects, and its administration may represent a future approach for prevention of IBD-induced intestinal strictures.
Keywords: CROHN'S DISEASE; EXTRACELLULAR MATRIX; FIBROGENESIS; FIBROSIS.
© Author(s) (or their employer(s)) 2024. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The Cleveland Clinic receives funds on her behalf from Celgene, Morphic, Pfizer, UCB, GB004 and Helmsley. SDH was consultant to Shionogi, Takeda and Guidepoint and receives research funding from the Crohn’s and Colitis Foundation and the American Society of Colon and Rectal Surgeons. CF receives speaker fees from UCB, Genentech, Sandoz and Janssen, and he is the consultant of Athos Therapeutics, Inc. FR is the consultant of Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene, Cowen, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jansen, Koutif, Metacrine, Morphic, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Takeda, Techlab, Thetis, UCB and 89Bio.
Figures



Comment in
-
It is in the matrix: a molecular clue to fibro-stenotic Crohn's disease.Gut. 2024 Jun 6;73(7):1045-1046. doi: 10.1136/gutjnl-2024-332016. Gut. 2024. PMID: 38378251 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
