Subcutaneous immunoglobulin replacement therapy in patients with immunodeficiencies - impact of drug packaging and administration method on patient reported outcomes
- PMID: 38378441
- PMCID: PMC10880328
- DOI: 10.1186/s12865-024-00608-0
Subcutaneous immunoglobulin replacement therapy in patients with immunodeficiencies - impact of drug packaging and administration method on patient reported outcomes
Abstract
Background: Here, the perspective of patients with primary and secondary immunodeficiency receiving subcutaneous immunoglobulin (SCIg) via introductory smaller size pre-filled syringes (PFS) or vials were compared.
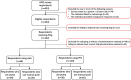
Methods: An online survey was conducted in Canada by the Association des Patients Immunodéficients du Québec (APIQ) (10/2020-03/2021). Survey questions included: reasons for choosing SCIg packaging and administration methods, training experiences, infusion characteristics, and switching methods. The survey captured structured patient-reported outcomes: treatment satisfaction and its sub-domains, symptom state, general health perception, and physical and mental function. Respondents using PFS were compared with vial users, overall and stratified by their administration method (pump or manual push).
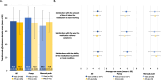
Results: Of the 132 total respondents, 66 respondents used vials, with 38 using a pump and 28 using manual push. PFS (5 and 10 mL sizes) were being used by 120 respondents, with 38 using a pump and 82 using manual push. PFS users were associated with a 17% lower median (interquartile range) SCIg dose (10 [8, 12] vs. 12 [9, 16] g/week, respectively), a significantly shorter infusion preparation time (15 [10, 20] vs. 15 [10, 30] mins, respectively), and a trend for shorter length of infusion (60 [35, 90] vs. 70 [48, 90] mins, respectively) compared with those on vials. Patient-reported treatment satisfaction scores were overall similar between vial and PFS users (including on the domains of effectiveness and convenience), except for a higher score for vials over PFS on the domain of global satisfaction (p=0.02).
Conclusions: Consistent with prescribing that reflects a recognition of less wastage, PFS users were associated with a significantly lower SCIg dose compared with vial users. PFS users were also associated with shorter pre-infusion times, reflecting simpler administration mechanics compared with vial users. Higher global satisfaction with treatment among vial users compared with PFS users was consistent with users being limited to smaller PFS size options in Canada during the study period. Patient experience on PFS is expected to improve with the introduction of larger PFS sizes. Overall, treatment satisfaction for SCIg remains consistently high with the introduction of PFS packaging compared with vials.
Keywords: Immunodeficiency; Manual push; Packaging method; Patient reported outcomes; Pre-filled syringes (PFS); Pump; Subcutaneous immunoglobulin (SCIg); Treatment satisfaction questionnaire for medication (TSQM); Vials.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
RM and XZ are full-time employees of and own stocks in CSL Behring. GS works for the APIQ, a patient advocacy group that facilitated the planning and execution of the survey, which was sponsored by CSL Behring. PB is an employee of Meridian HealthComms Ltd. PP is a former employee of CSL Behring. OL has no conflicts to declare.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

