Phosphorylation of plasma membrane H+-ATPase Thr881 participates in light-induced stomatal opening
- PMID: 38378616
- PMCID: PMC10879185
- DOI: 10.1038/s41467-024-45248-5
Phosphorylation of plasma membrane H+-ATPase Thr881 participates in light-induced stomatal opening
Abstract
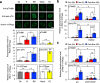
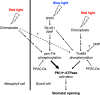
Plasma membrane (PM) H+-ATPase is crucial for light-induced stomatal opening and phosphorylation of a penultimate residue, Thr948 (pen-Thr, numbering according to Arabidopsis AHA1) is required for enzyme activation. In this study, a comprehensive phosphoproteomic analysis using guard cell protoplasts from Vicia faba shows that both red and blue light increase the phosphorylation of Thr881, of PM H+-ATPase. Light-induced stomatal opening and the blue light-induced increase in stomatal conductance are reduced in transgenic Arabidopsis plants expressing mutant AHA1-T881A in aha1-9, whereas the blue light-induced phosphorylation of pen-Thr is unaffected. Auxin and photosynthetically active radiation induce the phosphorylation of both Thr881 and pen-Thr in etiolated seedlings and leaves, respectively. The dephosphorylation of phosphorylated Thr881 and pen-Thr are mediated by type 2 C protein phosphatase clade D isoforms. Taken together, Thr881 phosphorylation, in addition of the pen-Thr phosphorylation, are important for PM H+-ATPase function during physiological responses, such as light-induced stomatal opening in Arabidopsis thaliana.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

