Gut dysbiosis induces the development of depression-like behavior through abnormal synapse pruning in microglia-mediated by complement C3
- PMID: 38378622
- PMCID: PMC10877840
- DOI: 10.1186/s40168-024-01756-6
Gut dysbiosis induces the development of depression-like behavior through abnormal synapse pruning in microglia-mediated by complement C3
Abstract
Background: Remodeling eubiosis of the gut microenvironment may contribute to preventing the occurrence and development of depression. Mounting experimental evidence has shown that complement C3 signaling is associated with the pathogenesis of depression, and disruption of the gut microbiota may be an underlying cause of complement system activation. However, the mechanism by which complement C3 participates in gut-brain crosstalk in the pathogenesis of depression remains unknown.
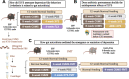
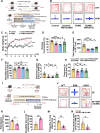
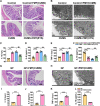
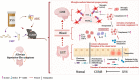
Results: In the present study, we found that chronic unpredictable mild stress (CUMS)-induced mice exhibited obvious depression-like behavior as well as cognitive impairment, which was associated with significant gut dysbiosis, especially enrichment of Proteobacteria and elevation of microbiota-derived lipopolysaccharides (LPS). In addition, peripheral and central complement C3 activation and central C3/CR3-mediated aberrant synaptic pruning in microglia have also been observed. Transplantation of gut microbiota from CUMS-induced depression model mice into specific pathogen-free and germ-free mice induced depression-like behavior and concomitant cognitive impairment in the recipient mice, accompanied by increased activation of the complement C3/CR3 pathway in the prefrontal cortex and abnormalities in microglia-mediated synaptic pruning. Conversely, antidepressants and fecal microbiota transplantation from antidepressant-treated donors improved depression-like behaviors and restored gut microbiome disturbances in depressed mice. Concurrently, inhibition of the complement C3/CR3 pathway, amelioration of abnormal microglia-mediated synaptic pruning, and increased expression of the synapsin and postsynaptic density protein 95 were observed. Collectively, our results revealed that gut dysbiosis induces the development of depression-like behaviors through abnormal synapse pruning in microglia-mediated by complement C3, and the inhibition of abnormal synaptic pruning is the key to targeting microbes to treat depression.
Conclusions: Our findings provide novel insights into the involvement of complement C3/CR3 signaling and aberrant synaptic pruning of chemotactic microglia in gut-brain crosstalk in the pathogenesis of depression. Video Abstract.
Keywords: Complement C3; Depression; Fecal microbiota transplantation; Gut microbiota; Microglia; Synaptic pruning.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- 2023M741396/China Postdoctoral Science Foundation
- 2021A1515010869; 2022A1515011699/the Guangdong Basic and Applied Basic Research Foundation
- 2021A1515010869; 2022A1515011699/the Guangdong Basic and Applied Basic Research Foundation
- 82074300, 82174278/National Natural Science Foundation of China
- 82074300, 82174278/National Natural Science Foundation of China
- 2020B1111100001/Key-Area Research and Development Program of Guangdong Province
- 2020B1111100001/Key-Area Research and Development Program of Guangdong Province
- 2021B1212040007/Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization
- 2021B1212040007/Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization
- 202201020052/Science and Technology Program of Guangzhou
- 202102010014/the Guangzhou Key Laboratory of Formula-Pattern of Traditional Chinese Medicine
- 202102010014/the Guangzhou Key Laboratory of Formula-Pattern of Traditional Chinese Medicine
- 201911/the Huang Zhendong Research Fund for Traditional Chinese Medicine of Jinan University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

