Clinical surveillance systems obscure the true cholera infection burden in an endemic region
- PMID: 38378884
- PMCID: PMC10957480
- DOI: 10.1038/s41591-024-02810-4
Clinical surveillance systems obscure the true cholera infection burden in an endemic region
Erratum in
-
Author Correction: Clinical surveillance systems obscure the true cholera infection burden in an endemic region.Nat Med. 2024 Sep;30(9):2693. doi: 10.1038/s41591-024-03177-2. Nat Med. 2024. PMID: 38982299 Free PMC article. No abstract available.
Abstract
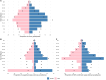
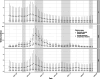
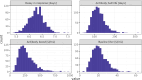
Our understanding of cholera transmission and burden largely relies on clinic-based surveillance, which can obscure trends, bias burden estimates and limit the impact of targeted cholera-prevention measures. Serological surveillance provides a complementary approach to monitoring infections, although the link between serologically derived infections and medically attended disease incidence-shaped by immunological, behavioral and clinical factors-remains poorly understood. We unravel this cascade in a cholera-endemic Bangladeshi community by integrating clinic-based surveillance, healthcare-seeking and longitudinal serological data through statistical modeling. Combining the serological trajectories with a reconstructed incidence timeline of symptomatic cholera, we estimated an annual Vibrio cholerae O1 infection incidence rate of 535 per 1,000 population (95% credible interval 514-556), with incidence increasing by age group. Clinic-based surveillance alone underestimated the number of infections and reported cases were not consistently correlated with infection timing. Of the infections, 4 in 3,280 resulted in symptoms, only 1 of which was reported through the surveillance system. These results impart insights into cholera transmission dynamics and burden in the epicenter of the seventh cholera pandemic, where >50% of our study population had an annual V. cholerae O1 infection, and emphasize the potential for a biased view of disease burden and infection risk when depending solely on clinical surveillance data.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

