Increases in cyclin A/Cdk activity and in PP2A-B55 inhibition by FAM122A are key mitosis-inducing events
- PMID: 38378890
- PMCID: PMC10943098
- DOI: 10.1038/s44318-024-00054-z
Increases in cyclin A/Cdk activity and in PP2A-B55 inhibition by FAM122A are key mitosis-inducing events
Abstract
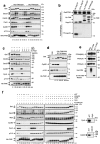
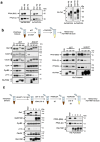
Entry into mitosis has been classically attributed to the activation of a cyclin B/Cdk1 amplification loop via a partial pool of this kinase becoming active at the end of G2 phase. However, how this initial pool is activated is still unknown. Here we discovered a new role of the recently identified PP2A-B55 inhibitor FAM122A in triggering mitotic entry. Accordingly, depletion of the orthologue of FAM122A in C. elegans prevents entry into mitosis in germline stem cells. Moreover, data from Xenopus egg extracts strongly suggest that FAM122A-dependent inhibition of PP2A-B55 could be the initial event promoting mitotic entry. Inhibition of this phosphatase allows subsequent phosphorylation of early mitotic substrates by cyclin A/Cdk, resulting in full cyclin B/Cdk1 and Greatwall (Gwl) kinase activation. Subsequent to Greatwall activation, Arpp19/ENSA become phosphorylated and now compete with FAM122A, promoting its dissociation from PP2A-B55 and taking over its phosphatase inhibition role until the end of mitosis.
Keywords: Arpp19; Cyclin A.; FAM122A; Mitosis; PP2A-B55.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Alvarez-Fernandez M, Sanchez-Martinez R, Sanz-Castillo B, Gan PP, Sanz-Flores M, Trakala M, Ruiz-Torres M, Lorca T, Castro A, Malumbres M. Greatwall is essential to prevent mitotic collapse after nuclear envelope breakdown in mammals. Proc Natal Acad Sci USA. 2013;110:17374–17379. doi: 10.1073/pnas.1310745110. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

