Stress-induced stenotic vascular remodeling via reduction of plasma omega-3 fatty acid metabolite 4-oxoDHA by noradrenaline
- PMID: 38378892
- PMCID: PMC10879168
- DOI: 10.1038/s41598-024-54867-3
Stress-induced stenotic vascular remodeling via reduction of plasma omega-3 fatty acid metabolite 4-oxoDHA by noradrenaline
Abstract
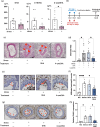
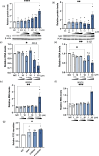
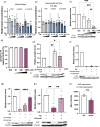
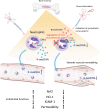
Stress has garnered significant attention as a prominent risk factor for inflammation-related diseases, particularly cardiovascular diseases (CVDs). However, the precise mechanisms underlying stress-driven CVDs remain elusive, thereby impeding the development of preventive and therapeutic strategies. To explore the correlation between plasma lipid metabolites and human depressive states, liquid chromatography-mass spectrometry (LC/MS) based analysis of plasma and the self-rating depression (SDS) scale questionnaire were employed. We also used a mouse model with restraint stress to study its effects on plasma lipid metabolites and stenotic vascular remodeling following carotid ligation. In vitro functional and mechanistic studies were performed using macrophages, endothelial cells, and neutrophil cells. We revealed a significant association between depressive state and reduced plasma levels of 4-oxoDHA, a specific omega-3 fatty acid metabolite biosynthesized by 5-lipoxygenase (LO), mainly in neutrophils. In mice, restraint stress decreased plasma 4-oxoDHA levels and exacerbated stenotic vascular remodeling, ameliorated by 4-oxoDHA supplementation. 4-oxoDHA enhanced Nrf2-HO-1 pathways, exerting anti-inflammatory effects on endothelial cells and macrophages. One of the stress hormones, noradrenaline, reduced 4-oxoDHA and the degraded 5-LO in neutrophils through the proteasome system, facilitated by dopamine D2-like receptor activation. Our study proposed circulating 4-oxoDHA levels as a stress biomarker and supplementation of 4-oxoDHA as a novel therapeutic approach for controlling stress-related vascular inflammation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case–control study. Lancet. 2004;364(9438):953–962. doi: 10.1016/S0140-6736(04)17019-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

