Impact of ageing on homologous and human-coronavirus-reactive antibodies after SARS-CoV-2 vaccination or infection
- PMID: 38378953
- PMCID: PMC10879087
- DOI: 10.1038/s41541-024-00817-z
Impact of ageing on homologous and human-coronavirus-reactive antibodies after SARS-CoV-2 vaccination or infection
Abstract
The endemic human coronaviruses (HCoVs) circulate worldwide yet remain understudied and unmitigated. The observation of elevated levels of HCoV reactive antibodies in COVID-19 patients highlights the urgent necessity of better understanding of HCoV specific immunity. Here, we characterized in-depth the de novo SARS-CoV-2 specific antibody responses and the boosting of HCoV-reactive antibodies after SARS-CoV-2 vaccination or infection in individuals up to 98 years old. All the vaccinees were home-dwelling with no documented SARS-CoV-2 infection before receiving the COVID-19 mRNA vaccine (BNT162b2). The first two vaccine doses elicited potent SARS-CoV-2 spike binding antibodies in individuals up to 80 years. The third dose largely boosted the previously low S2 domain binding and neutralizing antibodies in elderly 80-90 years old, but less so in those above 90 years. The endemic betacoronavirus (HKU1 and OC43) reactive antibodies were boosted in all vaccinees, although to a lesser extent in those above 80 years old. COVID-19 patients had potent elevation of alpha- and betacoronavirus (229E, NL63, HKU1 and OC43) reactive antibodies. In both patients and vaccinees, S2 domain specific antibody increases correlated with SARS-CoV-2 neutralizing and HCoV-reactive antibody responses in all ages, indicating S2 domain as a candidate for future universal coronavirus vaccine design.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



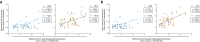

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

