Downregulation of praja2 restrains endocytosis and boosts tyrosine kinase receptors in kidney cancer
- PMID: 38379085
- PMCID: PMC10879500
- DOI: 10.1038/s42003-024-05823-4
Downregulation of praja2 restrains endocytosis and boosts tyrosine kinase receptors in kidney cancer
Abstract
Clear cell renal cell carcinoma (ccRCC) is the most common kidney cancer in the adult population. Late diagnosis, resistance to therapeutics and recurrence of metastatic lesions account for the highest mortality rate among kidney cancer patients. Identifying novel biomarkers for early cancer detection and elucidating the mechanisms underlying ccRCC will provide clues to treat this aggressive malignant tumor. Here, we report that the ubiquitin ligase praja2 forms a complex with-and ubiquitylates the AP2 adapter complex, contributing to receptor endocytosis and clearance. In human RCC tissues and cells, downregulation of praja2 by oncogenic miRNAs (oncomiRs) and the proteasome markedly impairs endocytosis and clearance of the epidermal growth factor receptor (EGFR), and amplifies downstream mitogenic and proliferative signaling. Restoring praja2 levels in RCC cells downregulates EGFR, rewires cancer cell metabolism and ultimately inhibits tumor cell growth and metastasis. Accordingly, genetic ablation of praja2 in mice upregulates RTKs (i.e. EGFR and VEGFR) and induces epithelial and vascular alterations in the kidney tissue.In summary, our findings identify a regulatory loop between oncomiRs and the ubiquitin proteasome system that finely controls RTKs endocytosis and clearance, positively impacting mitogenic signaling and kidney cancer growth.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

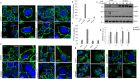

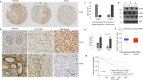
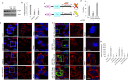



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

