Patient perspective on living with mild hemophilia in Germany: results from a nationwide survey
- PMID: 38379557
- PMCID: PMC10877726
- DOI: 10.3389/fmed.2024.1347024
Patient perspective on living with mild hemophilia in Germany: results from a nationwide survey
Abstract
Introduction: The disease burden and bleeding risk of patients with mild hemophilia may be underestimated. Their health-related quality of life (QoL) may be negatively impacted by insufficient treatment and bleed-related joint damage connected to a potentially delayed diagnosis.
Aim: This study aims to gain information on the care reality and QoL of patients aged ≥12 years with mild hemophilia in Germany.
Methods: An anonymous cross-sectional patient survey using standardized questionnaires was conducted in a validated electronic patient-reported outcome system. Medical specialists, hemophilia centers, patient organizations, and support groups across Germany invited the patients.
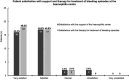
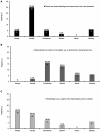
Results: A total of 43 patients (35 patients with hemophilia A, 5 patients with hemophilia B, and 3 patients for whom the information was missing) with a median age of 33 years were analyzed. The median age at diagnosis was 6.0 years (interquartile range [IQR] 2.0-15.0), and the median factor activity was 14.0% (IQR 12.0-25.0). Nearly 85% of the patients received factor concentrates in the past, and the most common reasons for the treatment were surgery or joint bleeding (each 65.6%). Half of the patients who provided feedback experienced complications during bleeding episodes. Prophylactic treatment with factor concentrates was rare (10.3%). The patients had minor problems regarding their health status.
Conclusion: Bleeding complications and joint bleeding, in particular, may be highly underestimated in patients with mild hemophilia, highlighting a medical need in this population. Patients with a potential benefit from prophylaxis need to be identified. Mild hemophilia has a negative impact on patients' QoL. Hemophilia centers satisfied the patients' needs. Further research is needed to address the current lack of awareness and improve adequate treatment in the future.
Keywords: care reality; hemophilia A; hemophilia B; joint bleeding; mild hemophilia; quality of life.
Copyright © 2024 Alesci, Goldmann, Halimeh, Holstein, Königs, Miesbach, Pfrepper and Olivieri.
Conflict of interest statement
RA has acted as a paid consultant to CSL Behring and has received funding for research carried out in this work. She has received funding from Takeda, Sobi, Octapharma, Grifols, and Novartis and meeting/congress support from Takeda, Sobi, and Bayer. Furthermore, RA acted as a paid consultant to Amgen, Bayer, BFSH, Grifols, LFB Pharma, Medlearning, Octapharma, onkowissen, STREAMED UP (Pfizer, Grifols), Sobi, and Takeda Pharma. GG reports receiving honoraria for lectures from Bayer, Biotest, Novo Nordisk, Octapharma, Roche, Sobi, and Takeda, and reimbursement for travel expenses from Biotest, Novo Nordisk, and Sobi. SH reports grants or contracts from Bayer Healthcare, Baxalta Innovations (now Takeda), Biotest, CSL Behring, Novo Nordisk Pharma, Octapharma, Pfizer Pharma. Further, SH has received honoraria from Bayer Healthcare, Baxalta Innovations (now Takeda), Biotest, CSL Behring, Novartis Pharma, Novo Nordisk Pharma, Octapharma, Pfizer Pharma, Roche Pharma; Swedish Orphan Biovitrum and consulting fees from Bayer Healthcare, Biotest, CSL Behring, Novo Nordisk Pharma, Octapharma, Chugai Pharma Germany, Swedish Orphan Biovitrum. KH reports grants for research or clinical studies (to institution): Bayer, CSL Behring, Novo Nordisk, Pfizer, Sobi and GWT/Roche, and honoraria for lectures or consultancy (to person): Bayer, BioMarin, Biotest, Chugai, CSL Behring, LFB, Novo Nordisk, Pfizer, Roche, Sobi and Takeda. KH has served as a consultant for Bayer, Biotest, CSL Behring, LFB, Novo Nordisk, Pfizer, Roche and Sobi, and in an advisory board for Octapharma. She has received meeting/congress support from Pfizer, Sobi, Takeda, Bayer, CSL Behring, Novo Nordisk and has served as a speaker for the Haemophilia Board of the GTH and is a member of the Ärztlicher Beirat of the Deutsche Hämophiliegesellschaft e.V. (DGH; Medical Advisory Board of the German Haemophilia Society e.V.). CK has received consulting fees or honoraria for lectures and meeting/congress support from BFSH, CSL Behring, MSD, Novo Nordisk, Roche/Chugai, Sobi/Sanofi, and Takeda. CK’s institution has received grants from Bayer Vital GmbH, Biotest, CSL Behring, Intersero, Novo Nordisk, Pfizer, Roche/Chugai, Sanofi/Sobi, Takeda, and the European Union (H2020) and federal funding. He is a member of the German Paediatric AIDS Society and Head of the German Haemophilia PUP Registry. WM has acted as a paid consultant to Bayer, BioMarin, Biotest, CSL Behring, Chugai, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, Regeneron, Roche, Sanofi, Sigilon, Sobi, Takeda/Shire, uniQure and has received research funding form Bayer, Biotest, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Sobi, Takeda/Shire. WM has received honoraria for lectures from Bayer, BioMarin, Biotest, CSL Behring, Chugai. LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Takeda/Shire and has received meeting/congress support from Bayer, BioMarin, Biotest, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, Takeda/Shire, uniQure. CP reports grants for studies and research from Chugai, Roche, LeoPharma, Zacros, and Takeda; and personal fees for lectures or consultancy from Alexion, Bayer, BioMarin, Chugai Pharma, Roche, CSL Behring, Leo Pharma, Novo Nordisk, Pfizer, BMS, Sanofi, Sobi, Takeda, and Zarcos. MO has received honoraria or consulting fees from Bayer, BioMarin, Biotest, Chugai, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, Stago and Takeda. MO has participated in DSMB/advisory boards from Bayer, Biomarin, Biotest, Chugai, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi and Takeda and has received meeting/travel support from Bayer, Biotest, CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi, Takeda. This study received funding from CSL Behring, Germany. The funder had the following involvement with the study: study design, the writing of this article (funding of medical writing support), and the decision to submit it for publication.
Figures

Similar articles
-
Defining the impact of hemophilia: the Academic Achievement in Children with Hemophilia Study.Pediatrics. 2001 Dec;108(6):E105. doi: 10.1542/peds.108.6.e105. Pediatrics. 2001. PMID: 11731632
-
Improving Hemophilia Care in Low- and Middle-Income Countries: Addressing Challenges and Enhancing Quality of Life.Cureus. 2024 Jun 21;16(6):e62817. doi: 10.7759/cureus.62817. eCollection 2024 Jun. Cureus. 2024. PMID: 39036274 Free PMC article. Review.
-
A Budget Impact Model of Hemophilia Bypassing Agent Prophylaxis Relative to Recombinant Factor VIIa On-Demand.J Manag Care Spec Pharm. 2016 Feb;22(2):149-57. doi: 10.18553/jmcp.2016.22.2.149. J Manag Care Spec Pharm. 2016. PMID: 27015254 Free PMC article.
-
The Patient Experience of Hemophilia and Human Immunodeficiency Virus: A Systematic Review of Qualitative Evidence.JBI Libr Syst Rev. 2012;10(58):4659-4668. doi: 10.11124/jbisrir-2012-435. JBI Libr Syst Rev. 2012. PMID: 27820530
-
Individualized prophylaxis for optimizing hemophilia care: can we apply this to both developed and developing nations?Thromb J. 2016 Oct 4;14(Suppl 1):32. doi: 10.1186/s12959-016-0096-y. eCollection 2016. Thromb J. 2016. PMID: 27766058 Free PMC article. Review.
References
-
- WFH World Federation of Hemophilia . Report on the annual global survey. Geneva: WFH; (2021).
-
- Barthels M, Bergmann F, Czwalinna A. Das Gerinnungskompendium. 2nd ed. Barthels M. Stuttgart: Thieme; (2012). doi: 10.1055/b-002-13417 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

