Clinical characteristics and risk factors analysis of 505 cases of infusion reactions in a tertiary hospital
- PMID: 38379900
- PMCID: PMC10876897
- DOI: 10.3389/fphar.2024.1292347
Clinical characteristics and risk factors analysis of 505 cases of infusion reactions in a tertiary hospital
Abstract
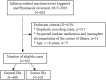
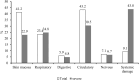
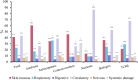
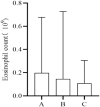
Background: The clinical characteristics and risk factors of infusion reactions (IRs) are inadequately described in clinical practice due to underreported cases. In the present study, we reported the current status of IRs based on an in-hospital pharmacovigilance database of a tertiary care hospital. Methods: Our study conducted a retrospective analysis of drug-induced IRs recorded at an in-hospital pharmacovigilance center between January 2015 to December 2019. The descriptive statistical analysis encompassed main causative agents, clinical manifestations, organ/system involvement and outcome. The severity of IRs was assessed with reference to the CTCAE version 5.0 criteria and we investigated risk factors associated with severe IRs. Results: During the study period, a total of 505 cases of inpatient drug-induced IRs were detected, of which 79.2% (400 cases) were classified as general IRs and 20.8% (105 cases) were categorized as severe IRs. The primary drugs responsible for these reactions were antibiotics (23%, 116 cases), with piperacillin sodium-sulbactam sodium being the most prevalent, followed by antineoplastic agents (18.4%, 93 cases) and traditional Chinese medicine injections (TCMIs) (12.9%, 65 cases). The administration of cefoperazone - sulbactam, mannatide, Shenqi Fuzheng, elemene, and diterpene ginkgolides meglumine resulted in a higher incidence of critical IRs. Among all cases of IRs, 43.2%, 41.2%, and 23.4% showed signs and symptoms of circulation, skin mucosa, and respiratory organs/systems, respectively. 9.1% of cases experienced systemic damage, while 7.1% and 5.9% of cases reported neurological and gastrointestinal related adverse reactions, respectively. The multivariate analysis revealed that alcohol consumption (OR = 2.389%, 95% CI 1.141-5.002, p = 0.021), age over 65 (OR = 1.814%, 95% CI 1.052-3.127, p = 0.032) and the utilization of contrast media (OR = 4.072%, 95% CI 1.903-8.713, p < 0.001) were identified as risk factors for the development of severe IRs. Conclusion: Understanding the clinical characteristics of IRs helps to implement effective pharmaceutical monitoring and appropriate preventive measures for susceptible populations with risk factors.
Keywords: clinical characteristics; infusion reactions; pharmacovigilance; risk factors; tertiary hospital.
Copyright © 2024 Yin, Wen, Wang, Wang, Kong, Wu, Meng, Ou, Wei and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pharmacovigilance of cutaneous adverse drug reactions in associations with drugs and medical conditions: a retrospective study of hospitalized patients.BMC Pharmacol Toxicol. 2022 Aug 10;23(1):62. doi: 10.1186/s40360-022-00603-4. BMC Pharmacol Toxicol. 2022. PMID: 35948985 Free PMC article.
-
Cetuximab infusion reactions: French pharmacovigilance database analysis.J Oncol Pharm Pract. 2013 Jun;19(2):130-7. doi: 10.1177/1078155212457965. Epub 2012 Nov 15. J Oncol Pharm Pract. 2013. PMID: 23154574
-
Eosinophilic drug reactions detected by a prospective pharmacovigilance programme in a tertiary hospital.Br J Clin Pharmacol. 2017 Feb;83(2):400-415. doi: 10.1111/bcp.13096. Epub 2016 Oct 12. Br J Clin Pharmacol. 2017. PMID: 27543764 Free PMC article.
-
Infusion-related reactions with pegloticase, a recombinant uricase for the treatment of chronic gout refractory to conventional therapy.J Clin Rheumatol. 2014 Dec;20(8):427-32. doi: 10.1097/RHU.0000000000000200. J Clin Rheumatol. 2014. PMID: 25417679 Free PMC article. Review.
-
[Research progress of adverse reactions of traditional Chinese medicine injections].Zhongguo Zhong Yao Za Zhi. 2014 Oct;39(20):3889-98. Zhongguo Zhong Yao Za Zhi. 2014. PMID: 25751935 Review. Chinese.
References
LinkOut - more resources
Full Text Sources

