Prevalence and clinical significance of potential drug-drug interactions among lung transplant patients
- PMID: 38379901
- PMCID: PMC10876870
- DOI: 10.3389/fphar.2024.1308260
Prevalence and clinical significance of potential drug-drug interactions among lung transplant patients
Abstract
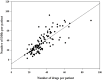
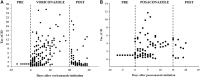
Background: Drug-drug interactions (DDIs) are a major but preventable cause of adverse drug reactions. There is insufficient information regarding DDIs in lung transplant recipients. Objective: This study aimed to determine the prevalence of potential DDIs (pDDIs) in intensive care unit (ICU) lung transplant recipients, identify the real DDIs and the most frequently implicated medications in this vulnerable population, and determine the risk factors associated with pDDIs. Methods: This retrospective cross-sectional study included lung transplant recipients from January 2018 to December 2021. Pertinent information was retrieved from medical records. All prescribed medications were screened for pDDIs using the Lexicomp® drug interaction software. According to this interaction software, pDDIs were classified as C, D, or X (C = monitor therapy, D = consider therapy modification, X = avoid combination). The Drug Interaction Probability Scale was used to determine the causation of DDIs. All statistical analysis was performed in SPSS version 26.0. Results: 114 patients were qualified for pDDI analysis, and total pDDIs were 4051. The most common type of pDDIs was category C (3323; 82.0%), followed by D (653; 16.1%) and X (75; 1.9%). Voriconazole and posaconazole were the antifungal medicine with the most genuine DDIs. Mean tacrolimus concentration/dose (Tac C/D) before or after co-therapy was considerably lower than the Tac C/D during voriconazole or posaconazole co-therapy (p < 0.001, p = 0.027). Real DDIs caused adverse drug events (ADEs) in 20 patients. Multivariable logistic regression analyses found the number of drugs per patient (OR, 1.095; 95% CI, 1.048-1.145; p < 0.001) and the Acute Physiology and Chronic Health Evaluation II (APACHE Ⅱ) score (OR, 1.097; 95% CI, 1.021-1.179; p = 0.012) as independent risk factors predicting category X pDDIs. Conclusion: This study revealed a high incidence of both potential and real DDIs in ICU lung transplant recipients. Immunosuppressive drugs administered with azole had a high risk of causing clinically significant interactions. The number of co-administered drugs and APACHE Ⅱ score were associated with an increased risk of category × drug interactions. Close monitoring of clinical and laboratory parameters is essential for ensuring successful lung transplantation and preventing adverse drug events associated with DDIs.
Keywords: intensive care unit; lung transplant; potential drug-drug interactions; tacrolimus; voriconazole.
Copyright © 2024 Zhang, Ma, Chen, Hu, Chen, Chen, Huang and Dai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abbas A., Al-Shaibi S., Sankaralingam S., Awaisu A., Kattezhathu V. S., Wongwiwatthananukit S., et al. (2022). Determination of potential drug–drug interactions in prescription orders dispensed in a community pharmacy setting using Micromedex® and Lexicomp®: a retrospective observational study. Int. J. Clin. Pharm. 44, 348–356. 10.1007/s11096-021-01346-8 - DOI - PubMed
LinkOut - more resources
Full Text Sources

