Individual ingredients of NP-101 (Thymoquinone formula) inhibit SARS-CoV-2 pseudovirus infection
- PMID: 38379905
- PMCID: PMC10876831
- DOI: 10.3389/fphar.2024.1291212
Individual ingredients of NP-101 (Thymoquinone formula) inhibit SARS-CoV-2 pseudovirus infection
Abstract
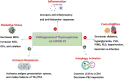
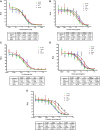
Thymoquinone TQ, an active ingredient of Nigella Sativa, has been shown to inhibit COVID-19 symptoms in clinical trials. Thymoquinone Formulation (TQF or NP-101) is developed as a novel enteric-coated medication derivative from Nigella Sativa. TQF consists of TQ with a favorable concentration and fatty acids, including palmitic, oleic, and linoleic acids. In this study, we aimed to investigate the roles of individual ingredients of TQF on infection of SARS-CoV-2 variants in-vitro, by utilizing Murine Leukemia Virus (MLV) based pseudovirus particles. We demonstrated that NP-101, TQ, and other individual ingredients, including oleic, linoleic, and palmitic acids inhibited SARS-CoV-2 infection in the MLV-based pseudovirus model. A large, randomized phase 2 study of NP-101 is planned in outpatient COVID-19 patients.
Keywords: COVID-19; COVID-19 and anti-viral agents; Coronavirus; TQ formula; Thymoquinone TQ; fatty acids.
Copyright © 2024 Maen, Gok Yavuz, Mohamed, Esmail, Lu, Mohamed, Azmi, Kaseb, Kasseb, Li, Gocio, Kocak, Selim, Ma and Kaseb.
Conflict of interest statement
Author JL was employed by Codex BioSolutions Inc. Authors MoK, OK, and MG were employed by Novatek Pharmaceuticals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Abdelrahim M., Esmail A., Al Saadi N., Zsigmond E., Al Najjar E., Bugazia D., et al. (2022). Thymoquinone's antiviral effects: it is time to be proven in the covid-19 pandemic era and its Omicron variant surge. Front. Pharmacol. 13, 848676. PubMed PMID: 35462919. 10.3389/fphar.2022.848676 - DOI - PMC - PubMed
-
- Ashraf S., Ashraf S., Ashraf M., Imran M. A., Kalsoom L., Siddiqui U. N., et al. (2020). Therapeutic efficacy of Honey and Nigella sativa against COVID-19: a multi-center randomized controlled clinical trial (HNS-COVID-PK). medRxiv. Available at: 10.1101/2020.10.30.20217364. - DOI
-
- Bencheqroun H., Ahmed Y., Kocak M., Villa E., Barrera C., Mohiuddin M., et al. (2022). A randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety and efficacy of ThymoQuinone formula (TQF) for treating outpatient SARS-CoV-2. Pathogens 11 (5), 551. 10.3390/pathogens11050551 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

