Thermoregulatory response in juvenile Hippocampus erectus: Effect of magnitude and rate of thermal increase on metabolism and antioxidative defence
- PMID: 38380062
- PMCID: PMC10877557
- DOI: 10.1002/ece3.10977
Thermoregulatory response in juvenile Hippocampus erectus: Effect of magnitude and rate of thermal increase on metabolism and antioxidative defence
Abstract
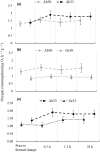
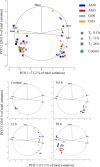
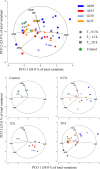
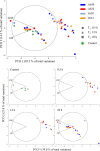
Behavioural, physiological and biochemical mechanisms constitute the adaptive capacities that allow marine ectotherms to explore the environment beyond their thermal optimal. Limitations to the efficiency of these mechanisms define the transition from moderate to severe thermal stress, and serve to characterise the thermoregulatory response in the zone of thermal tolerance. We selected a tropical population of Hippocampus erectus to describe the timing of the physiological and biochemical mechanisms in response to the following increments in water temperature: (i) 4°C abrupt (26-30°C in <5 min); (ii) 7°C abrupt (26-33°C); (iii) 4°C gradual (1°C every 3 h) and (iv) 7°C gradual (1.5°C every 3 h). The routine metabolic rate (Rrout) of juvenile H. erectus was measured immediately before and after 0.5, 12 and 28 h of being exposed to each thermal treatment. Samples of muscle and abdominal organs were taken to quantify indicators of aerobic and anaerobic metabolism and antioxidant enzymes and oxidative stress at each moment throughout exposure. Results showed a full thermoregulatory response within 0.5 h: Rrout increased in direct correspondence with both the magnitude and rate of thermal increase; peroxidised lipids rapidly accumulated before the antioxidant defence was activated and early lactate concentrations suggested an immediate, yet temporary, reduction in aerobic scope. After 12 h, Rrout had decreased in sea horses exposed to 30°C, but not to 33°C, where Rrout continued high until the end of trials. Within 28 h of thermal exposure, all metabolite and antioxidant defence indicators had been restored to control levels (26°C). These findings testify to the outstanding thermal plasticity of H. erectus and explain their adjustment to rapid fluctuations in ambient temperature. Such features, however, do not protect this tropical population from the deleterious effects of chronic exposure to temperatures that have been predicted for the future.
Keywords: antioxidative defence; ocean warming; routine metabolism; sea horses; thermal biology; thermal tolerance.
© 2024 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no relevant financial or non‐financial interests to disclose and declare that they have no conflict of interest.
Figures
Similar articles
-
Effect of a gradually increasing temperature on the behavioural and physiological response of juvenile Hippocampus erectus: Thermal preference, tolerance, energy balance and growth.J Therm Biol. 2019 Oct;85:102406. doi: 10.1016/j.jtherbio.2019.102406. Epub 2019 Aug 31. J Therm Biol. 2019. PMID: 31657747
-
Transcriptomic response in thermally challenged seahorses Hippocampus erectus: The effect of magnitude and rate of temperature change.Comp Biochem Physiol B Biochem Mol Biol. 2022 Oct-Dec;262:110771. doi: 10.1016/j.cbpb.2022.110771. Epub 2022 Jun 9. Comp Biochem Physiol B Biochem Mol Biol. 2022. PMID: 35691555
-
Changes in clinicomorphometrical findings, lipid profiles, hepatorenal indices and oxidant/antioxidant status as thermoregulatory adaptive mechanisms in poikilothermic Dabb lizard (Uromastyx aegyptia).Sci Rep. 2023 Feb 28;13(1):3409. doi: 10.1038/s41598-023-30184-z. Sci Rep. 2023. PMID: 36854728 Free PMC article.
-
Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals.Comp Biochem Physiol A Mol Integr Physiol. 2002 Aug;132(4):739-61. doi: 10.1016/s1095-6433(02)00045-4. Comp Biochem Physiol A Mol Integr Physiol. 2002. PMID: 12095860 Review.
-
Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals.Naturwissenschaften. 2001 Apr;88(4):137-46. doi: 10.1007/s001140100216. Naturwissenschaften. 2001. PMID: 11480701 Review.
References
-
- Aksnes, A. , & Njaa, L. R. (1981). Catalase, glutathione peroxidase and superoxide dismutase in different fish species. Comparative Biochemistry and Physiology. B, 69, 893–896. 10.1016/0305-0491(81)90402-8 - DOI
-
- Almeida‐Val, V. M. F. , Oliveira, A. R. , Silva, M. d. N. P. d. , Ferreira‐Nozawa, M. S. , Araújo, R. M. , Val, A. L. , & Nozawa, S. R. (2011). Anoxia‐ and hypoxia‐induced expression of LDH‐A in the Amazon Oscar, Astronotus crassipinis . Genetics and Molecular Biology, 34, 315–322. 10.1590/S1415-47572011000200025 - DOI - PMC - PubMed
-
- Anderson, M. J. (2017). Permutational multivariate analysis of variance (PERMANOVA). In Wiley StatsRef: Statistics reference online. John Wiley & Sons, Ltd. 10.1002/9781118445112.stat07841 - DOI
LinkOut - more resources
Full Text Sources

