Circulating Nucleic Acids in Colorectal Cancer: Diagnostic and Prognostic Value
- PMID: 38380073
- PMCID: PMC10878755
- DOI: 10.1155/2024/9943412
Circulating Nucleic Acids in Colorectal Cancer: Diagnostic and Prognostic Value
Abstract
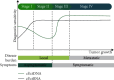
Colorectal cancer (CRC) is the third most prevalent cancer in the world and the fourth leading cause of cancer-related mortality. DNA (cfDNA/ctDNA) and RNA (cfRNA/ctRNA) in the blood are promising noninvasive biomarkers for molecular profiling, screening, diagnosis, treatment management, and prognosis of CRC. Technological advancements that enable precise detection of both genetic and epigenetic abnormalities, even in minute quantities in circulation, can overcome some of these challenges. This review focuses on testing for circulating nucleic acids in the circulation as a noninvasive method for CRC detection, monitoring, detection of minimal residual disease, and patient management. In addition, the benefits and drawbacks of various diagnostic techniques and associated bioinformatics tools have been detailed.
Copyright © 2024 Somayeh Igder et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Recent Technologies towards Diagnostic and Therapeutic Applications of Circulating Nucleic Acids in Colorectal Cancers.Int J Mol Sci. 2024 Aug 9;25(16):8703. doi: 10.3390/ijms25168703. Int J Mol Sci. 2024. PMID: 39201393 Free PMC article. Review.
-
A new approach to epigenome-wide discovery of non-invasive methylation biomarkers for colorectal cancer screening in circulating cell-free DNA using pooled samples.Clin Epigenetics. 2018 Apr 16;10:53. doi: 10.1186/s13148-018-0487-y. eCollection 2018. Clin Epigenetics. 2018. PMID: 29686738 Free PMC article.
-
Circulating cell-free nucleic acids as biomarkers in colorectal cancer screening and diagnosis - an update.Expert Rev Mol Diagn. 2019 Jun;19(6):477-498. doi: 10.1080/14737159.2019.1613891. Epub 2019 May 21. Expert Rev Mol Diagn. 2019. PMID: 31046485 Review.
-
Somatic copy number alteration and fragmentation analysis in circulating tumor DNA for cancer screening and treatment monitoring in colorectal cancer patients.J Hematol Oncol. 2022 Sep 2;15(1):125. doi: 10.1186/s13045-022-01342-z. J Hematol Oncol. 2022. PMID: 36056434 Free PMC article.
-
Cell-Free DNA as a Diagnostic Blood-Based Biomarker for Colorectal Cancer: A Systematic Review.J Surg Res. 2019 Apr;236:184-197. doi: 10.1016/j.jss.2018.11.029. Epub 2018 Dec 21. J Surg Res. 2019. PMID: 30694754
References
-
- Naini M. A., Kavousipour S., Hasanzarini M., Nasrollah A., Monabati A., Mokarram P. O6-methyguanine-DNA methyl transferase (MGMT) promoter methylation in serum DNA of Iranian patients with colorectal cancer. Asian Pacific Journal of Cancer Prevention . 2018;18(5):1223–1227. doi: 10.22034/APJCP.2018.19.5.1223. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

