Phase II Trial of FOLFIRINOX in Advanced Biliary Tract Cancer
- PMID: 38380191
- PMCID: PMC10878013
- DOI: 10.7759/cureus.52656
Phase II Trial of FOLFIRINOX in Advanced Biliary Tract Cancer
Abstract
Introduction: Biliary tract cancers (BTCs), characterized by poor prognosis and limited treatment options, are increasingly prevalent malignancies with a five-year survival rate of less than 20% for advanced-stage disease. The standard first-line chemotherapy combining gemcitabine and cisplatin offers modest survival benefits, necessitating the exploration of more effective therapies. This study reports the results of a single-arm, open-label, phase 2 trial assessing the efficacy and safety of fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFIRINOX) as a first-line treatment for metastatic or locally advanced unresectable BTC.
Methods: Patients aged ≥18 with measurable disease and adequate organ function were enrolled, receiving biweekly FOLFIRINOX for up to 12 cycles with follow-up imaging every four cycles. The primary endpoint was the overall response rate (ORR), with progression-free survival (PFS), overall survival (OS), and safety as secondary endpoints.
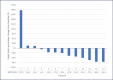
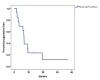
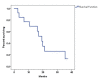
Results: Thirteen patients were enrolled from December 2016 to September 2021 before early termination due to slow accrual and the emergence of immunotherapy. The ORR was 54%, with a disease control rate of 77%. Median PFS and OS were 6.8 and 19.25 months, respectively. Grade 3/4 toxicities were predominantly hematologic, with neutropenia being the most common severe adverse event.
Conclusion: The trial suggests that FOLFIRINOX is a potentially effective first-line therapy for unresectable or metastatic BTC with a manageable safety profile. However, the early termination of the study and the introduction of immunotherapy warrant further research to confirm these findings.
Keywords: advanced biliary tract cancer; cancer of gallbladder; cholangiocarcinoma; folfirinox chemotherapy; phase ii.
Copyright © 2024, Bazarbashi et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Banales JM, Cardinale V, Carpino G, et al. Nat Rev Gastroenterol Hepatol. 2016;13:261–280. - PubMed
-
- Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. Bridgewater J, Galle PR, Khan SA, et al. J Hepatol. 2014;60:1268–1289. - PubMed
-
- Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. Valle J, Wasan H, Palmer DH, et al. N Engl J Med. 2010;362:1273–1281. - PubMed
LinkOut - more resources
Full Text Sources
