Alleviating sleep disturbances and modulating neuronal activity after ischemia: Evidence for the benefits of zolpidem in stroke recovery
- PMID: 38380702
- PMCID: PMC10880125
- DOI: 10.1111/cns.14637
Alleviating sleep disturbances and modulating neuronal activity after ischemia: Evidence for the benefits of zolpidem in stroke recovery
Abstract
Aims: Sleep disorders are prevalent among stroke survivors and impede stroke recovery, yet they are still insufficiently considered in the management of stroke patients, and the mechanisms by which they occur remain unclear. There is evidence that boosting phasic GABA signaling with zolpidem during the repair phase improves stroke recovery by enhancing neural plasticity; however, as a non-benzodiazepine hypnotic, the effects of zolpidem on post-stroke sleep disorders remain unclear.
Method: Transient ischemic stroke in male rats was induced with a 30-minute middle cerebral artery occlusion. Zolpidem or vehicle was intraperitoneally delivered once daily from 2 to 7 days after the stroke, and the electroencephalogram and electromyogram were recorded simultaneously. At 24 h after ischemia, c-Fos immunostaining was used to assess the effect of transient ischemic stroke and acute zolpidem treatment on neuronal activity.
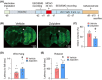
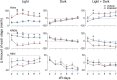
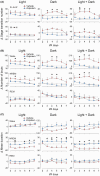
Results: In addition to the effects on reducing brain damage and mitigating behavioral deficits, repeated zolpidem treatment during the subacute phase of stroke quickly ameliorated circadian rhythm disruption, alleviated sleep fragmentation, and increased sleep depth in ischemic rats. Immunohistochemical staining showed that in contrast to robust activation in para-infarct and some remote areas by 24 h after the onset of focal ischemia, the activity of the ipsilateral suprachiasmatic nucleus, the biological rhythm center, was strongly suppressed. A single dose of zolpidem significantly upregulated c-Fos expression in the ipsilateral suprachiasmatic nucleus to levels comparable to the contralateral side.
Conclusion: Stroke leads to suprachiasmatic nucleus dysfunction. Zolpidem restores suprachiasmatic nucleus activity and effectively alleviates post-stroke sleep disturbances, indicating its potential to promote stroke recovery.
Keywords: MCAO; c-Fos; circadian rhythm; sleep disorder; suprachiasmatic nucleus; zolpidem.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no competing interests. <Sun, Fengyan> is an Editorial Board member of CNS Neuroscience and Therapeutics and a co‐author of this article. To minimize bias, she was excluded from all editorial decision‐making related to the acceptance of this article for publication.
Figures





References
-
- Dabrowska‐Bender M, Milewska M, Golabek A, Duda‐Zalewska A, Staniszewska A. The impact of ischemic cerebral stroke on the quality of life of patients based on clinical, social, and psychoemotional factors. J Stroke Cerebrovasc Dis. 2017;26(1):101‐107. - PubMed
-
- Khaku AS, Tadi P. Cerebrovascular disease. In StatPearls. Statpearls Publishing; 2021. - PubMed
-
- Viale L, Catoira NP, Di Girolamo G, Gonzalez CD. Pharmacotherapy and motor recovery after stroke. Expert Rev Neurother. 2018;18(1):65‐82. - PubMed
-
- Mortensen JK, Andersen G. Potential role of selective serotonin reuptake inhibitors in improving functional outcome after stroke. CNS Drugs. 2018;32(10):895‐903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2023YFC2306500/National Key Research and Development Program of China
- 81771430/National Natural Science Foundation of China
- 81971239/National Natural Science Foundation of China
- 82020108014/National Natural Science Foundation of China
- 2021ZD0203400/National Major Project of China Science and Technology Innovation 2030 for Brain Science and Brain-Inspired Technology
LinkOut - more resources
Full Text Sources
Medical

